NOV/CCN3 induces cartilage protection by inhibiting PI3K/AKT/mTOR pathway
- PMID: 31454155
- PMCID: PMC6815824
- DOI: 10.1111/jcmm.14621
NOV/CCN3 induces cartilage protection by inhibiting PI3K/AKT/mTOR pathway
Abstract
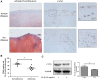
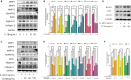
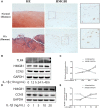
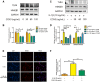
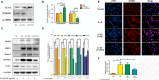
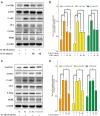
Osteoarthritis (OA), an age-related degenerative joint disease, is pathologically characterized by articular cartilage degeneration and synovial inflammation. Nephroblastoma overexpressed (NOV or CCN3), a matricellular protein, is a primary member of the CCN family (Cyr61, Ctgf, NOV) of proteins and is involved in various inflammatory disorders. Previous studies reported that CCN3 might play a therapeutic role in OA. However, the underlying mechanism remains unclear. In this study, we confirmed the expression of CCN3 was decreased in human and rat OA articular cartilage. Recombinant CCN3 ameliorated the IL-1β-induced matrix catabolism, as demonstrated by MMP1, MMP3, MMP13, ADAMTS5 and iNOS expression, in vitro. In addition, the degradation of cartilage matrix such as collagen 2 and aggrecan could be reversed by CCN3. Furthermore, we found CCN3 promoted autophagy as Atg5, Beclin1 and LC3-II expression were increased. High-mobility group box 1 was negatively correlated with CCN3 in IL-1β-induced osteoarthritis responses, and HMGB1 is involved in the protective effect of CCN3 in OA. Moreover, CCN3 overexpression decreased the expression of HMGB1 and reversed the IL-1β induced MMPs production. Additionally, recombinant CCN3 or CCN3 overexpression attenuated the activation of PI3K/AKT/mTOR pathway induced by IL-1β. Our study presents new mechanisms of CCN3 in osteoarthritis and indicates that CCN3 can serve as a novel potential therapeutic target for osteoarthritis.
Keywords: CCN3; HMGB1; IL-1β; MMPs; osteoarthritis.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there is no conflict of interest.
Figures

References
-
- Tong W, Zeng Y, Chow D, et al. Wnt16 attenuates osteoarthritis progression through a PCP/JNK‐mTORC1‐PTHrP cascade. Ann Rheum Dis. 2019;78:551‐561. - PubMed
-
- O'Neill TW, McCabe PS, McBeth J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract Res Clin Rheumatol. 2018;32:312‐326. - PubMed
-
- Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27:359‐364. - PubMed
-
- Verma P, Dalal K. ADAMTS‐4 and ADAMTS‐5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507‐3514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

