Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial
- PMID: 31454263
- PMCID: PMC6909845
- DOI: 10.1164/rccm.201903-0557OC
Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial
Abstract
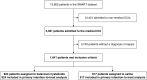
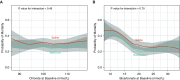
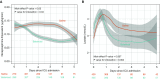
Rationale: Administration of intravenous crystalloid solutions is a fundamental therapy for sepsis, but the effect of crystalloid composition on patient outcomes remains unknown.Objectives: To compare the effect of balanced crystalloids versus saline on 30-day in-hospital mortality among critically ill adults with sepsis.Methods: Secondary analysis of patients from SMART (Isotonic Solutions and Major Adverse Renal Events Trial) admitted to the medical ICU with an International Classification of Diseases, 10th Edition, Clinical Modification System code for sepsis, using multivariable regression to control for potential confounders.Measurements and Main Results: Of 15,802 patients enrolled in SMART, 1,641 patients were admitted to the medical ICU with a diagnosis of sepsis. A total of 217 patients (26.3%) in the balanced crystalloids group experienced 30-day in-hospital morality compared with 255 patients (31.2%) in the saline group (adjusted odds ratio [aOR], 0.74; 95% confidence interval [CI], 0.59-0.93; P = 0.01). Patients in the balanced group experienced a lower incidence of major adverse kidney events within 30 days (35.4% vs. 40.1%; aOR, 0.78; 95% CI, 0.63-0.97) and a greater number of vasopressor-free days (20 ± 12 vs. 19 ± 13; aOR, 1.25; 95% CI, 1.02-1.54) and renal replacement therapy-free days (20 ± 12 vs. 19 ± 13; aOR, 1.35; 95% CI, 1.08-1.69) compared with the saline group.Conclusions: Among patients with sepsis in a large randomized trial, use of balanced crystalloids was associated with a lower 30-day in-hospital mortality compared with use of saline.Clinical trial registered with www.clinicaltrials.gov (NCT02444988).
Keywords: balanced crystalloids; lactated Ringer’s; saline; sepsis; septic shock.
Figures
Comment in
-
Balanced Crystalloids or 0.9% Saline in Sepsis. Beyond Reasonable Doubt?Am J Respir Crit Care Med. 2019 Dec 15;200(12):1456-1458. doi: 10.1164/rccm.201908-1669ED. Am J Respir Crit Care Med. 2019. PMID: 31513748 Free PMC article. No abstract available.
-
Balanced Crystalloids in Sepsis: Are We There Yet?Am J Respir Crit Care Med. 2020 May 1;201(9):1162-1163. doi: 10.1164/rccm.202001-0077LE. Am J Respir Crit Care Med. 2020. PMID: 31972095 Free PMC article. No abstract available.
-
Should We Avoid Saline in Sepsis? It's Probably Too Early to Definitively Conclude.Am J Respir Crit Care Med. 2020 May 1;201(9):1161-1162. doi: 10.1164/rccm.201912-2479LE. Am J Respir Crit Care Med. 2020. PMID: 31972097 Free PMC article. No abstract available.
-
Reply to Gueret et al. and to Hammond et al.Am J Respir Crit Care Med. 2020 May 1;201(9):1163-1164. doi: 10.1164/rccm.202001-0073LE. Am J Respir Crit Care Med. 2020. PMID: 31972099 Free PMC article. No abstract available.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. - PubMed
-
- Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, et al. PRISM Investigators. Early, goal-directed therapy for septic shock: a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. 2016. Crit Care Med. 2017;45:486–552. - PubMed
-
- Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27:179–188. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

