Structural Basis for Allosteric Modulation of Class B G Protein-Coupled Receptors
- PMID: 31454292
- PMCID: PMC7092389
- DOI: 10.1146/annurev-pharmtox-010919-023301
Structural Basis for Allosteric Modulation of Class B G Protein-Coupled Receptors
Abstract
Recent advances in our understanding of the structure and function of class B G protein-coupled receptors (GPCRs) provide multiple opportunities for targeted development of allosteric modulators. Given the pleiotropic signaling patterns emanating from these receptors in response to a variety of natural agonist ligands, modulators have the potential to sculpt the responses to meet distinct needs of different groups of patients. In this review, we provide insights into how this family of GPCRs differs from the rest of the superfamily, how orthosteric agonists bind and activate these receptors, the potential for allosteric modulators to interact with various regions of these targets, and the allosteric influence of endogenous proteins on the pharmacology of these receptors, all of which are important considerations when developing new therapies.
Keywords: GPCRs; RAMPs; allosteric modulation; biased agonism; structure function.
Figures
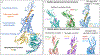
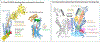
References
-
- Traynor K 2018. FDA approves licensing of erenumab-aooe to prevent migraine. Am. J. Health. Syst. Pharm 75:929–30 - PubMed
-
- Liang YL, Khoshouei M, Glukhova A, Furness SGB, Zhao P, et al. 2018. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555:121–5 - PubMed
-
- Hollenstein K, Kean J, Bortolato A, Cheng RK, Dore AS, et al. 2013. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 499:438–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

