Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors
- PMID: 31454760
- PMCID: PMC6705619
- DOI: 10.1212/NXI.0000000000000604
Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors
Abstract
Objective: To report the induction of anti-Ma2 antibody-associated paraneoplastic neurologic syndrome (Ma2-PNS) in 6 patients after treatment with immune checkpoint inhibitors (ICIs). We also analyzed (1) patient clinical features compared with a cohort of 44 patients who developed Ma2-PNS without receiving ICI treatment and (2) the frequency of neuronal antibody detection before and after ICI implementation.
Methods: Retrospective nationwide study of all patients with Ma2-PNS developed during ICI treatment between 2017 and 2018.
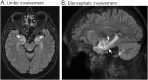
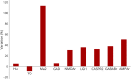
Results: Our series of patients included 5 men and 1 woman (median age, 63 years). The patients were receiving nivolumab (n = 3), pembrolizumab (n = 2), or a combination of nivolumab and ipilimumab (n = 1) for treatment of neoplasms that included lung (n = 4) and kidney (n = 1) cancers and pleural mesothelioma (n = 1). Clinical syndromes comprised a combination of limbic encephalitis and diencephalitis (n = 3), isolated limbic encephalitis (n = 2), and a syndrome characterized by ophthalmoplegia and head drop (n = 1). No significant clinical difference was observed between our 6 patients and the overall cohort of Ma2-PNS cases. Post-ICI Ma2-PNS accounted for 35% of the total 17 Ma2-PNS diagnosed in our center over the 2017-2018 biennium. Eight cases had been detected in the preceding biennium 2015-2016, corresponding to a 112% increase of Ma2-PNS frequency since the implementation of ICIs in France. Despite ICI withdrawal and immunotherapy, 4/6 patients died, and the remaining 2 showed a moderate to severe disability.
Conclusions: We show a clear association between ICI use and increased diagnosis of Ma2-PNS. Physicians need to be aware that ICIs can trigger Ma2-PNS because clinical presentation can be challenging.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017;13:755–763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
