β-Glucose-1,6-Bisphosphate Stabilizes Pathological Phophomannomutase2 Mutants In Vitro and Represents a Lead Compound to Develop Pharmacological Chaperones for the Most Common Disorder of Glycosylation, PMM2-CDG
- PMID: 31454904
- PMCID: PMC6747070
- DOI: 10.3390/ijms20174164
β-Glucose-1,6-Bisphosphate Stabilizes Pathological Phophomannomutase2 Mutants In Vitro and Represents a Lead Compound to Develop Pharmacological Chaperones for the Most Common Disorder of Glycosylation, PMM2-CDG
Abstract
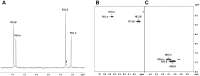
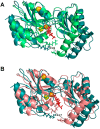
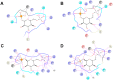
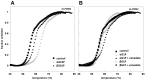
A large number of mutations causing PMM2-CDG, which is the most frequent disorder of glycosylation, destabilize phosphomannomutase2. We looked for a pharmacological chaperone to cure PMM2-CDG, starting from the structure of a natural ligand of phosphomannomutase2, α-glucose-1,6-bisphosphate. The compound, β-glucose-1,6-bisphosphate, was synthesized and characterized via 31P-NMR. β-glucose-1,6-bisphosphate binds its target enzyme in silico. The binding induces a large conformational change that was predicted by the program PELE and validated in vitro by limited proteolysis. The ability of the compound to stabilize wild type phosphomannomutase2, as well as frequently encountered pathogenic mutants, was measured using thermal shift assay. β-glucose-1,6-bisphosphate is relatively resistant to the enzyme that specifically hydrolyses natural esose-bisphosphates.
Keywords: PMM2-CDG; glucose-1,6-bisphosphate; pharmacological chaperone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Citro V., Cimmaruta C., Liguori L., Viscido G., Cubellis M.V., Andreotti G. A mutant of phosphomannomutase1 retains full enzymatic activity, but is not activated by IMP: Possible implications for the disease PMM2-CDG. PLoS ONE. 2017;12:e0189629. doi: 10.1371/journal.pone.0189629. - DOI - PMC - PubMed
-
- Van Schaftingen E., Jaeken J. Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 1995;377:318–320. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

