ELQ-331 as a prototype for extremely durable chemoprotection against malaria
- PMID: 31455339
- PMCID: PMC6712883
- DOI: 10.1186/s12936-019-2921-9
ELQ-331 as a prototype for extremely durable chemoprotection against malaria
Abstract
Background: The potential benefits of long-acting injectable chemoprotection (LAI-C) against malaria have been recently recognized, prompting a call for suitable candidate drugs to help meet this need. On the basis of its known pharmacodynamic and pharmacokinetic profiles after oral dosing, ELQ-331, a prodrug of the parasite mitochondrial electron transport inhibitor ELQ-300, was selected for study of pharmacokinetics and efficacy as LAI-C in mice.
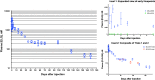
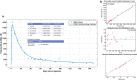
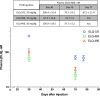
Methods: Four trials were conducted in which mice were injected with a single intramuscular dose of ELQ-331 or other ELQ-300 prodrugs in sesame oil with 1.2% benzyl alcohol; the ELQ-300 content of the doses ranged from 2.5 to 30 mg/kg. Initial blood stage challenges with Plasmodium yoelii were used to establish the model, but the definitive study measure of efficacy was outcome after sporozoite challenge with a luciferase-expressing P. yoelii, assessed by whole-body live animal imaging. Snapshot determinations of plasma ELQ-300 concentration ([ELQ-300]) were made after all prodrug injections; after the highest dose of ELQ-331 (equivalent to 30 mg/kg ELQ-300), both [ELQ-331] and [ELQ-300] were measured at a series of timepoints from 6 h to 5½ months after injection.
Results: A single intramuscular injection of ELQ-331 outperformed four other ELQ-300 prodrugs and, at a dose equivalent to 30 mg/kg ELQ-300, protected mice against challenge with P. yoelii sporozoites for at least 4½ months. Pharmacokinetic evaluation revealed rapid and essentially complete conversion of ELQ-331 to ELQ-300, a rapidly achieved (< 6 h) and sustained (4-5 months) effective plasma ELQ-300 concentration, maximum ELQ-300 concentrations far below the estimated threshold for toxicity, and a distinctive ELQ-300 concentration versus time profile. Pharmacokinetic modeling indicates a high-capacity, slow-exchange tissue compartment which serves to accumulate and then slowly redistribute ELQ-300 into blood, and this property facilitates an extremely long period during which ELQ-300 concentration is sustained above a minimum fully-protective threshold (60-80 nM).
Conclusions: Extrapolation of these results to humans predicts that ELQ-331 should be capable of meeting and far-exceeding currently published duration-of-effect goals for anti-malarial LAI-C. Furthermore, the distinctive pharmacokinetic profile of ELQ-300 after treatment with ELQ-331 may facilitate durable protection and enable protection for far longer than 3 months. These findings suggest that ELQ-331 warrants consideration as a leading prototype for LAI-C.
Keywords: Chemoprevention; Chemoprotection; Intra-muscular; Long-acting; Malaria; Plasmodium; Prodrug; Prophylaxis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Landovitz RJ, Li S, Grinsztejn B, Dawood H, Liu AY, Magnus M, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi: 10.1371/journal.pmed.1002690. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 14S-RCS001/U.S. Department of Veterans Affairs
- I01 BX001421/BX/BLRD VA/United States
- IK6 BX004857/BX/BLRD VA/United States
- T32AI007472/National Institute of Allergy and Infectious Diseases
- AI100569/NH/NIH HHS/United States
- T32 AI007472/AI/NIAID NIH HHS/United States
- number i01 BX003312/U.S. Department of Veterans Affairs
- R01 AI141412/AI/NIAID NIH HHS/United States
- IS1 BX003568/BX/BLRD VA/United States
- R01 AI100569/AI/NIAID NIH HHS/United States
- Log # PR130649; Contract # W81XWH-14-1-0447/U.S. Department of Defense
- I01 BX003312/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

