Is that a real oocyst? Insectary establishment and identification of Plasmodium falciparum oocysts in midguts of Anopheles mosquitoes fed on infected human blood in Tororo, Uganda
- PMID: 31455343
- PMCID: PMC6712792
- DOI: 10.1186/s12936-019-2922-8
Is that a real oocyst? Insectary establishment and identification of Plasmodium falciparum oocysts in midguts of Anopheles mosquitoes fed on infected human blood in Tororo, Uganda
Abstract
Background: The human infectious reservoir for malaria consists of individuals capable of infecting mosquitoes. Oocyst prevalence and density are typical indicators of human infectivity to mosquitoes. However, identification of oocysts is challenging, particularly in areas of low malaria transmission intensity where few individuals may infect mosquitoes, and infected mosquitoes tend to have few oocysts. Here, features that differentiate oocysts from other oocyst-like in mosquito midguts are explained and illustrated. In addition, the establishment and maintenance of infrastructure to perform malaria transmission experiments is described. This work may support other initiatives to set up membrane feeding infrastructure and guide oocyst detection in low transmission settings.
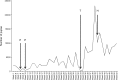
Methods: In 2014, an insectary was developed and equipped in Tororo district, Uganda. A colony of Anopheles gambiae s.s. mosquitoes (Kisumu strain) was initiated to support infectivity experiments from participants enrolled in a large cohort study. Venous blood drawn from participants who were naturally infected with malaria parasites was used for membrane feeding assays, using 60-80 mosquitoes per experiment. Approximately 9-10 days after feeding, mosquitoes were dissected, and midguts were stained in mercurochrome and examined by light microscopy for Plasmodium falciparum oocysts and similar structures. In supportive experiments, different staining procedures were compared using in vitro cultured parasites.
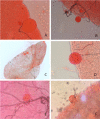
Results: A stable colony of the Kisumu strain of An. gambiae s.s. was achieved, producing 5000-10,000 adult mosquitoes on a weekly basis. Challenges due to temperature fluctuations, mosquito pathogens and pests were successfully overcome. Oocysts were characterized by: presence of malaria pigment, clearly defined edge, round shape within the mosquito midgut or on the peripheral tissue and always attached to the epithelium. The main distinguishing feature between artifacts and mature oocysts was the presence of defined pigment within the oocysts.
Conclusions: Oocysts may be mistaken for other structures in mosquito midguts. Distinguishing real oocysts from oocyst-like structures may be challenging for inexperienced microscopists due to overlapping features. The characteristics and guidelines outlined here support identification of oocysts and reliable detection at low oocyst densities. Practical advice on sustaining a healthy mosquito colony for feeding experiments is provided. Following the reported optimization, the established infrastructure in Tororo allows assessments of infectivity of naturally infected parasite carriers.
Keywords: Gametocyte; Malaria; Oocyst; Plasmodium falciparum; Transmission.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- WHO . World malaria report 2018. Geneva: World Health Organization; 2018.
-
- WHO . Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

