A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae
- PMID: 31455739
- PMCID: PMC6744904
- DOI: 10.1073/pnas.1907833116
A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae
Abstract
Hypervirulent Klebsiella pneumoniae (hvKp) is globally disseminating as a community-acquired pathogen causing life-threatening infections in healthy individuals. The fact that a dose as little as 50 bacteria is lethal to mice illustrates the dramatic increase of virulence associated with hvKp strains compared with classical K. pneumoniae (cKp) strains, which require lethal doses greater than 107 bacteria. Until recently, these virulent strains were mostly antibiotic-susceptible. However, multidrug-resistant (MDR) hvKp strains have been emerging, spawning a new generation of hypervirulent "superbugs." The mechanisms of hypervirulence are not fully defined, but overproduction of capsular polysaccharide significantly impedes host clearance, resulting in increased pathogenicity of hvKp strains. While there are more than 80 serotypes of K. pneumoniae, the K1 and K2 serotypes cause the vast majority of hypervirulent infections. Therefore, a glycoconjugate vaccine targeting these 2 serotypes could significantly reduce hvKp infection. Conventionally, glycoconjugate vaccines are manufactured using intricate chemical methodologies to covalently attach purified polysaccharides to carrier proteins, which is widely considered to be technically challenging. Here we report on the recombinant production and analytical characterization of bioconjugate vaccines, enzymatically produced in glycoengineered Escherichia coli cells, against the 2 predominant hypervirulent K. pneumoniae serotypes, K1 and K2. The K. pneumoniae bioconjugates are immunogenic and efficacious, protecting mice against lethal infection from 2 hvKp strains, NTUH K-2044 and ATCC 43816. This preclinical study constitutes a key step toward preventing further global dissemination of hypervirulent MDR hvKp strains.
Keywords: bioconjugation; glycoconjugate; hypervirulent Klebsiella pneumoniae; vaccine.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: M.F.F. and C.M.H. have a financial stake in VaxNewMo, a for-profit entity developing bioconjugate vaccines against Streptococcus pneumoniae and Klebsiella pneumoniae using patented technology derived from the data presented in this and other published manuscripts.
Figures
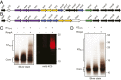
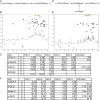

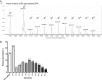
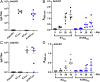

References
-
- Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention , Antibiotic Resistance Threats in the United States (Centers for Disease Control and Prevention, Atlanta, GA, 2013).
-
- Cheng D. L., Liu Y. C., Yen M. Y., Liu C. Y., Wang R. S., Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch. Intern. Med. 151, 1557–1559 (1991). - PubMed
-
- Liu Y. C., Cheng D. L., Lin C. L., Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch. Intern. Med. 146, 1913–1916 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

