Obinutuzumab plus fludarabine and cyclophosphamide in previously untreated, fit patients with chronic lymphocytic leukemia: a subgroup analysis of the GREEN study
- PMID: 31455851
- PMCID: PMC7214269
- DOI: 10.1038/s41375-019-0554-1
Obinutuzumab plus fludarabine and cyclophosphamide in previously untreated, fit patients with chronic lymphocytic leukemia: a subgroup analysis of the GREEN study
Abstract
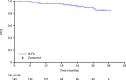
GREEN (NCT01905943) is a nonrandomized, open-label, single-arm, phase 3b study investigating the safety and efficacy of obinutuzumab alone or in combination with chemotherapy in chronic lymphocytic leukemia (CLL). We report the preplanned subgroup analysis of 140 previously untreated, fit CLL patients who received obinutuzumab plus fludarabine and cyclophosphamide (G-FC). The primary endpoint was safety and tolerability. Efficacy was the secondary endpoint. Obinutuzumab 1000 mg was administered intravenously on Day (D)1 (dose split D1‒2), D8 and D15 of Cycle (C)1, and D1 of C2-6 (28-day cycles). Standard intravenous/oral doses of fludarabine and cyclophosphamide were administered on D1-3 of C1-6. Overall, 87.1% of patients experienced grade ≥ 3 adverse events (AEs), including neutropenia (67.1%) and thrombocytopenia (17.1%). Serious AEs were experienced by 42.1% of patients. Rates of grade ≥ 3 infusion-related reactions and infections were 19.3% and 15.7%, respectively. Overall response rate was observed in 90.0%, with 46.4% of patients achieving complete response (CR; including CR with incomplete marrow recovery). Minimal residual disease negativity rates were 64.3% in peripheral blood and 35.7% in bone marrow (intent-to-treat analysis). After a median observation time of 25.6 months, 2 year progression-free survival was 91%. Frontline G-FC represents a promising treatment option for fit patients with CLL.
Conflict of interest statement
FB reports: consultancy, honoraria, and speakers bureau for Roche, Novartis, Janssen, Abbvie, Gilead, and Mundipharma; research funding from Roche, Celgene, Karyospharm, and Takeda; AZ reports: consultancy for Novartis and Janssen; speakers bureau for Novartis; J-LM and ET reports: research funding from Roche; KT reports: employment and equity ownership for Roche; SR reports: employment for Roche; EG reports: former employment for Roche; SB reports consulting fees from Roche and AbbVie; research funding from Roche, AbbVie, Janssen, and Celgene; and honoraria from Roche, AbbVie, Novartis, Janssen, and Becton Dickinson; SS reports: honoraria, consultancy, advisory board membership, travel support, research funding, and speakers bureau for AbbVie, Amgen, Celgene, Genentech, Gilead, GlaxoSmithKline, Janssen, Novartis, Pharmacyclics, and Roche; RF reports: consultancy and speakers bureau for Roche, Janssen, Gilead, Amgen, Celgene, BMS, Sandoz, Novartis, and Abbvie; VL reports: honoraria for Abbvie, Roche, Gilead, Janssen, BMS, Novartis, and Servier; advisory board membership for Abbvie, Roche, Gilead, Janssen, Novartis, and Servier; speakers bureau for Abbvie and Janssen; MT reports: advisory board membership, speaker bureau, and research funding from Roche; WK reports honoraria from Roche; GC, AC, and MT report no conflicts of interest.
Figures

References
-
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

