An analysis of genetic heterogeneity in untreated cancers
- PMID: 31455892
- PMCID: PMC6816333
- DOI: 10.1038/s41568-019-0185-x
An analysis of genetic heterogeneity in untreated cancers
Abstract
Genetic intratumoural heterogeneity is a natural consequence of imperfect DNA replication. Any two randomly selected cells, whether normal or cancerous, are therefore genetically different. Here, we review the different forms of genetic heterogeneity in cancer and re-analyse the extent of genetic heterogeneity within seven types of untreated epithelial cancers, with particular regard to its clinical relevance. We find that the homogeneity of predicted functional mutations in driver genes is the rule rather than the exception. In primary tumours with multiple samples, 97% of driver-gene mutations in 38 patients were homogeneous. Moreover, among metastases from the same primary tumour, 100% of the driver mutations in 17 patients were homogeneous. With a single biopsy of a primary tumour in 14 patients, the likelihood of missing a functional driver-gene mutation that was present in all metastases was 2.6%. Furthermore, all functional driver-gene mutations detected in these 14 primary tumours were present among all their metastases. Finally, we found that individual metastatic lesions responded concordantly to targeted therapies in 91% of 44 patients. These analyses indicate that the cells within the primary tumours that gave rise to metastases are genetically homogeneous with respect to functional driver-gene mutations, and we suggest that future efforts to develop combination therapies have the potential to be curative.
Conflict of interest statement
Competing interests statement
K.W.K. and B.V. are founders of Personal Genome Diagnostics and Thrive and advisors of Sysmex, Eisai, CAGE, Neophore. B.V. is also an advisor to Nexus. These companies and others have licensed technologies related to the work described in this paper from Johns Hopkins University. Some of these licenses are associated with equity or royalty payments to K.W.K. and B.V. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. Other authors declare no competing interests.
Figures

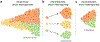
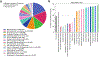
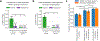


References
-
- Heppner GH Tumor Heterogeneity. Cancer Res 44, 2259–2265 (1984). - PubMed
-
- Martincorena I & Campbell PJ Somatic mutation in cancer and normal cells. Science 349, 1483–1489 (2015). - PubMed
-
- Rosenthal R, McGranahan N, Herrero J & Swanton C Deciphering Genetic Intratumor Heterogeneity and Its Impact on Cancer Evolution. Annu. Rev. Cancer Biol 1, 223–240 (2017).
-
- Engelman JA et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

