Helper-Dependent Adenovirus Transduces the Human and Rat Retina but Elicits an Inflammatory Reaction When Delivered Subretinally in Rats
- PMID: 31456426
- PMCID: PMC6854516
- DOI: 10.1089/hum.2019.159
Helper-Dependent Adenovirus Transduces the Human and Rat Retina but Elicits an Inflammatory Reaction When Delivered Subretinally in Rats
Abstract
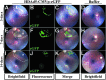
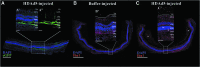
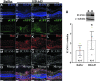
The identification of >100 genes causing inherited retinal degeneration and the promising results of recent gene augmentation trials have led to an increase in the number of studies investigating the preclinical efficacy of viral-mediated gene transfer. Despite success using adeno-associated viruses, many disease-causing genes, such as ABCA4 or USH2A, are too large to fit into these vectors. One option for large gene delivery is the family of integration-deficient helper-dependent adenoviruses (HDAds), which efficiently transduce postmitotic neurons. However, HDAds have been shown in other organ systems to elicit an immune response, and the immunogenicity of HDAds in the retina has not been characterized. In this study, HDAd serotype 5 (HDAd5) was found to successfully transduce rod and cone photoreceptors in ex vivo human retinal organ cultures. The ocular inflammatory response to subretinal injection of the HDAd5 was evaluated using a rat model. Subretinal injection of HDAd5 carrying cytomegalovirus promoter-driven enhanced green fluorescent protein (HDAd5-CMVp-eGFP) elicited a robust inflammatory response by 3 days postinjection. This reaction included vitreous infiltration of ionized calcium-binding adapter molecule 1 (Iba1)-positive monocytes and increased expression of the proinflammatory protein, intercellular adhesion molecule 1 (ICAM-1). By 7 days postinjection, most Iba1-positive infiltrates migrated into the neural retina and ICAM-1 expression was significantly increased compared with buffer-injected control eyes. At 14 days postinjection, Iba1-positive cells persisted in the retinas of HDAd5-injected eyes, and there was thinning of the outer nuclear layer. Subretinal injection of an empty HDAd5 virus was used to confirm that the inflammatory response was in response to the HDAd5 vector and not due to eGFP-induced overexpression cytotoxicity. Subretinal injection of lower doses of HDAd5 dampened the inflammatory response, but also eGFP expression. Despite their larger carrying capacity, further work is needed to elucidate the inflammatory pathways involved and to identify an immunomodulation paradigm sufficient for safe and effective transfer of large genes to the retina using HDAd5.
Keywords: helper-dependent adenovirus; inflammation; retina.
Conflict of interest statement
No competing financial interests exist for any of the authors of this article.
Figures





References
-
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 - PubMed
-
- Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol 2018;193:130–142 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

