Host Microbe Interactions in the Lactating Mammary Gland
- PMID: 31456777
- PMCID: PMC6701204
- DOI: 10.3389/fmicb.2019.01863
Host Microbe Interactions in the Lactating Mammary Gland
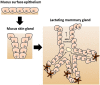
Abstract
The bacteria present in human milk constitute the human milk microbiome (hMM). Both the older culture-based work and the more recent studies using molecular detection of bacterial DNA have reached similar conclusions: the hMM mostly consists of commensal staphylococci such as Staphylococcus epidermidis, and streptococci. The prevalence of other bacterial groups such lactobacilli varies widely, while the abundance and prevalence of bifidobacteria is generally low. Recently, the hMM became accepted as a part of a physiologically normal state with suggested potential health benefits. Most research on the hMM has focused on its composition and potential effect on the breastfed infant. A major role as a microbiome inoculum for the infant gut has been proposed, but remains to be clearly demonstrated. Herein, we also discuss the emerging connection between the hMM and mammary gland physiology and lactation. Similarities between the mammary gland and mucosal interfaces are considerable, and in particular mucosal-like immune attributes of mammary gland. The potential role of hMM-host interactions in the mammary gland in maternal health is explored with a primary focus on lactational mastitis.
Keywords: breastfeeding; human milk; lactation; mastitis; microbiome; mucosal surface.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

