Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells
- PMID: 31456802
- PMCID: PMC6700333
- DOI: 10.3389/fimmu.2019.01891
Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells
Abstract
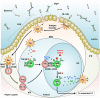
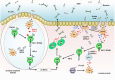
The intestinal mucosa is a physiological barrier for most microbes, including both commensal bacteria and invading pathogens. Under homeostatic conditions, immunoglobulin A (IgA) is the major immunoglobulin isotype in the intestinal mucosa. Microbes stimulate the production of IgA, which controls bacterial translocation and neutralizes bacterial toxins at the intestinal mucosal surface. In the intestinal mucosa, dendritic cells (DCs), specialized antigen-presenting cells, regulate both T-cell-dependent (TD) and -independent (TI) immune responses. The intestinal DCs are a heterogeneous population that includes unique subsets that induce IgA synthesis in B cells. The characteristics of intestinal DCs are strongly influenced by the microenvironment, including the presence of commensal bacterial metabolites and epithelial cell-derived soluble factors. In this review, we summarize the ontogeny, classification, and function of intestinal DCs and how the intestinal microenvironment conditions DCs and their precursors to become the mucosal phenotype, in particular to regulate IgA production, after they arrive at the intestine. Understanding the mechanism of IgA synthesis could provide insights for designing effective mucosal vaccines.
Keywords: IgA; commensal bacteria; conditioning; dendritic cells; intestine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

