Cryptococcus neoformans Induces MCP-1 Release and Delays the Death of Human Mast Cells
- PMID: 31456952
- PMCID: PMC6700240
- DOI: 10.3389/fcimb.2019.00289
Cryptococcus neoformans Induces MCP-1 Release and Delays the Death of Human Mast Cells
Abstract
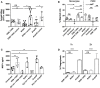
Cryptococcosis, caused by the basidiomycete Cryptococcus neoformans, is a life-threatening disease affecting approximately one million people per year worldwide. Infection can occur when C. neoformans cells are inhaled by immunocompromised people. In order to establish infection, the yeast must bypass recognition and clearance by immune cells guarding the tissue. Using in vitro infections, we characterized the role of mast cells (MCs) in cryptococcosis. We found that MCs recognize C. neoformans and release inflammatory mediators such as tryptase and cytokines. From the latter group MCs released mainly CCL-2/MCP-1, a strong chemoattractant for monocytic cells. We demonstrated that supernatants of infected MCs recruit monocytes but not neutrophils. During infection with C. neoformans, MCs have a limited ability to kill the yeast depending on the serotype. C. neoformans, in turn, modulates the lifespan of MCs both, by presence of its polysaccharide capsule and by secreting soluble modulators. Taken together, MCs might have important contributions to fungal clearance during early stages of cryptocococis where these cells regulate recruitment of monocytes to mucosal tissues.
Keywords: Cryptococcus neoformans; fungi; innate immunity; mast cells; monocyte chemoattractant protein 1/CCL-2; monocytes.
Figures

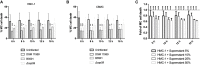
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

