RGD-Modified Nano-Liposomes Encapsulated Eptifibatide with Proper Hemocompatibility and Cytotoxicity Effect
- PMID: 31457055
- PMCID: PMC6697844
- DOI: 10.21859/ijb.2008
RGD-Modified Nano-Liposomes Encapsulated Eptifibatide with Proper Hemocompatibility and Cytotoxicity Effect
Abstract
Background: Eptifibatide (Integrilin®) is a hepta-peptide drug which specifically prevents the aggregation of activated platelets. The peptide drugs are encapsulated into nanolipisomes in order to decreasing their side effects and improving their half-life and bioavailability.
Objectives: In this study, the in vitro cytotoxicity and hemocompatibility of RGD-modified nano-liposomes (RGD-MNL) encapsulated a highly potent antiplatelet drug (eptifibatide) was investigated.
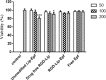
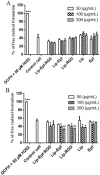
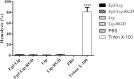
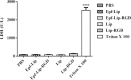
Material and methods: RGD-MNL encapsulated eptifibatide was prepared using lipid film hydration and freeze/thawing method. The morphology and size distribution (about 90 nm) of RGD-MNL were characterized using transmission electron microscopy (TEM). The in-vitro cytotoxicity of nano-liposomes was examined using the MTT, LDH release and reactive oxygen species (ROS) generation assays. The effect of RGD-MNL on red blood cells (RBC) was investigated using hemolysis and LDH release assays.
Results: The results revealed that RGD-MNL had no significant cytotoxic effect on HeLa and HUVEC cell lines, and also no ROS generation increase in the cells. In addition, the adverse effect of RGD-MNL on LDH release and membrane integrity of RBC was not observed.
Conclusions: In conclusion, the recommended RGD-MNL formulations have not any significant cytotoxicity on normal cells or RBC and have potential for protecting and enhancing the activity of antiplatelet drugs.
Keywords: Cytotoxicity; Eptifibatide; Liposomes; Materials Testing.
Figures


References
-
- Chang K, Chiu J-J. Clinical applications of nanotechnology in atherosclerotic diseases. Curr Nanosci. 2005;1(2):107–115.
-
- Tcheng JE, O’Shea JC. Eptifibatide: a potent inhibitor of the platelet receptor integrin, glycoprotein IIb/IIIa. Expert Opin Investig Drugs. 1999;8(11):1893–1905. pmid: 11139832 - PubMed
-
- Addeo R, Faiola V, Guarrasi R, Montella L, Vincenzi B, Capasso E, et al. Liposomal pegylated doxorubicin plus vinorelbine combination as first-line chemotherapy for metastatic breast cancer in elderly women > or = 65 years of age. Cancer Chemother Pharmacol. 2008;62(2):285–292. doi: 10.1007/s00280-007-0605-6 pmid: 17922275 - PubMed
-
- Torchilin VP. Targeting of drugs and drug carriers within the cardiovascular system Adv Drug Delivery Rev. 1995;17 75–101.
-
- Mohammadian M, Farzampanah L, Behtash-oskouie A, Majdi S, Mohseni G, Imandar M, et al. A biosensor for detect nitrite (NO2-) and hydroxylamine (nh2oh) by using of hydroxylamine oxidase and modified electrode with ZnO nanoparticles. Int J Electrochem Sci. 2013;8(9):11215–11227.
LinkOut - more resources
Full Text Sources
