Molecular Mechanism for the Hofmeister Effect Derived from NMR and DSC Measurements on Barnase
- PMID: 31457155
- PMCID: PMC6640789
- DOI: 10.1021/acsomega.6b00223
Molecular Mechanism for the Hofmeister Effect Derived from NMR and DSC Measurements on Barnase
Abstract
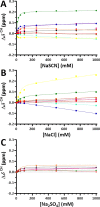
The effects of sodium thiocyanate, sodium chloride, and sodium sulfate on the ribonuclease barnase were studied using differential scanning calorimetry (DSC) and NMR. Both measurements reveal specific and saturable binding at low anion concentrations (up to 250 mM), which produces localized conformational and energetic effects that are unrelated to the Hofmeister series. The binding of sulfate slows intramolecular motions, as revealed by peak broadening in 13C heteronuclear single quantum coherence spectroscopy. None of the anions shows significant binding to hydrophobic groups. Above 250 mM, the DSC results are consistent with the expected Hofmeister effects in that the chaotropic anion thiocyanate destabilizes barnase. In this higher concentration range, the anions have approximately linear effects on protein NMR chemical shifts, with no evidence for direct interaction of the anions with the protein surface. We conclude that the effects of the anions on barnase are mediated by solvent interactions. The results are not consistent with the predictions of the preferential interaction, preferential hydration, and excluded volume models commonly used to describe Hofmeister effects. Instead, they suggest that the Hofmeister anion effects on both stability and solubility of barnase are due to the way in which the protein interacts with water molecules, and in particular with water dipoles, which are more ordered around sulfate anions and less ordered around thiocyanate anions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Cox W. M.; Wolfenden J. H. Proc. R. Soc. London, Ser. A 1934, 145, 475–488. 10.1098/rspa.1934.0113. - DOI
- Tielrooij K. J.; Garcia-Araez N.; Bonn M.; Bakker H. J. Science 2010, 328, 1006–1009. 10.1126/science.1183512. - DOI - PubMed
- dos Santos A. P.; Levin Y. Faraday Discuss. 2013, 160, 75–87. 10.1039/C2FD20067H. - DOI - PubMed
- Collins K. D.; Wasabaugh M. W. Q. Rev. Biophys. 1985, 18, 323–422. 10.1017/S0033583500005369. - DOI - PubMed
- Marcus Y. Chem. Rev. 2009, 109, 1346–1370. 10.1021/cr8003828. - DOI - PubMed
- Jenkins H. D. B.; Marcus Y. Chem. Rev. 1995, 95, 2695–2724. 10.1021/cr00040a004. - DOI
- Heydweiller A. Ann. Phys. 1910, 338, 145–185. 10.1002/andp.19103381108. - DOI
-
- Hofmeister F. Arch. Exp. Pathol. Pharmakol. 1888, 25, 1–30. 10.1007/BF01838161. - DOI
-
- Von Hippel P. H.; Wong K. J. Biol. Chem. 1965, 240, 3909–3923. - PubMed
-
- Von Hippel P. H.; Schleich T. Acc. Chem. Res. 1969, 2, 257–265. 10.1021/ar50021a001. - DOI
-
- Zhang Y.; Cremer P. S. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 15249–15253. 10.1073/pnas.0907616106. - DOI - PMC - PubMed
- Ries-Kautt M. M.; Ducruix A. F. J. Biol. Chem. 1989, 264, 745–748. - PubMed
- Baldwin R. L. Biophys. J. 1996, 71, 2056–2063. 10.1016/S0006-3495(96)79404-3. - DOI - PMC - PubMed
- Curtis R. A.; Prausnitz J. M.; Blanch H. W. Biotechnol. Bioeng. 1997, 57, 11–21. 10.1002/(SICI)1097-0290(19980105)57:1<11::AID-BIT2>3.0.CO;2-Y. - DOI - PubMed
- Arakawa T.; Timasheff S. N. Biochemistry 1982, 21, 6545–6552. 10.1021/bi00268a034. - DOI - PubMed
- Santoro M. M.; Liu Y.; Khan S. M. A.; Hou L.-X.; Bolen D. W. Biochemistry 1992, 31, 5278–5283. 10.1021/bi00138a006. - DOI - PubMed
LinkOut - more resources
Full Text Sources

