Heterogeneous Metal-Free Hydrogenation over Defect-Laden Hexagonal Boron Nitride
- PMID: 31457200
- PMCID: PMC6640807
- DOI: 10.1021/acsomega.6b00315
Heterogeneous Metal-Free Hydrogenation over Defect-Laden Hexagonal Boron Nitride
Abstract
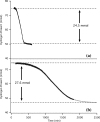
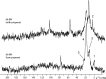
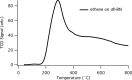
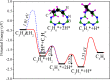
Catalytic hydrogenation is an important process used for the production of everything from foods to fuels. Current heterogeneous implementations of this process utilize metals as the active species. Until recently, catalytic heterogeneous hydrogenation over a metal-free solid was unknown; implementation of such a system would eliminate the health, environmental, and economic concerns associated with metal-based catalysts. Here, we report good hydrogenation rates and yields for a metal-free heterogeneous hydrogenation catalyst as well as its unique hydrogenation mechanism. Catalytic hydrogenation of olefins was achieved over defect-laden h-BN (dh-BN) in a reactor designed to maximize the defects in h-BN sheets. Good yields (>90%) and turnover frequencies (6 × 10-5-4 × 10-3) were obtained for the hydrogenation of propene, cyclohexene, 1,1-diphenylethene, (E)- and (Z)-1,2-diphenylethene, octadecene, and benzylideneacetophenone. Temperature-programmed desorption of ethene over processed h-BN indicates the formation of a highly defective structure. Solid-state NMR (SSNMR) measurements of dh-BN with high and low propene surface coverages show four different binding modes. The introduction of defects into h-BN creates regions of electronic deficiency and excess. Density functional theory calculations show that both the alkene and hydrogen-bond order are reduced over four specific defects: boron substitution for nitrogen (BN), vacancies (VB and VN), and Stone-Wales defects. SSNMR and binding-energy calculations show that VN are most likely the catalytically active sites. This work shows that catalytic sites can be introduced into a material previously thought to be catalytically inactive through the production of defects.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Bullock M. R.Front Matter. In Catalysis without Precious Metals; Bullock M. R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010; pp I–XVIII.
-
- Irandoust S.; Edvardsson J. Poisoning of nickel-based catalysts in fat hydrogenation. J. Am. Oil Chem. Soc. 1993, 70, 1149–1156. 10.1007/BF02632158. - DOI
-
- Anwa F.; Kazi T. G.; Saleem R.; Bhanger M. I. Rapid determination of some trace metals in several oils and fats. Grasas Aceites (Sevilla, Spain) 2004, 55, 160–168. 10.3989/gya.2004.v55.i2.162. - DOI
-
- Cempel M.; Nikel G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. http://www.pjoes.com/abstracts/2006/Vol15/No03/02.html.
LinkOut - more resources
Full Text Sources
Other Literature Sources

