Design Principle and Loss Engineering for Photovoltaic-Electrolysis Cell System
- PMID: 31457482
- PMCID: PMC6641131
- DOI: 10.1021/acsomega.7b00012
Design Principle and Loss Engineering for Photovoltaic-Electrolysis Cell System
Abstract
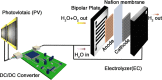
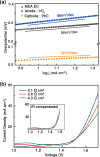
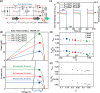
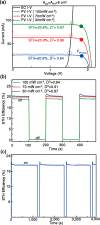
The effects of exchange current density, Tafel slope, system resistance, electrode area, light intensity, and solar cell efficiency were systematically decoupled at the converter-assisted photovoltaic-water electrolysis system. This allows key determinants of overall efficiency to be identified. On the basis of this model, 26.5% single-junction GaAs solar cell was combined with a membrane-electrode-assembled electrolysis cell (EC) using the dc/dc converting technology. As a result, we have achieved a solar-to-hydrogen conversion efficiency of 20.6% on a prototype scale and demonstrated light intensity tracking optimization to maintain high efficiency. We believe that this study will provide design principles for combining solar cells, ECs, and new catalysts and can be generalized to other solar conversion chemical devices while minimizing their power loss during the conversion of electrical energy into fuel.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Gahleitner G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrogen Energy 2013, 38, 2039–2061. 10.1016/j.ijhydene.2012.12.010. - DOI
-
- Pinaud B. A.; Benck J. D.; Seitz L. C.; Forman A. J.; Chen Z.; Deutsch T. G.; James B. D.; Baum K. N.; Baum G. N.; Ardo S.; Wang H.; Miller E.; Jaramillo T. F. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. 10.1039/c3ee40831k. - DOI
-
- Linsebigler A. L.; Lu G.; Yates J. T. Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. 10.1021/cr00035a013. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
