Singlet and Triplet Excited State Energy Ordering in Cyclopenta-Fused Polycyclic Aromatic Hydrocarbons (CP-PAHs) Suitable for Energy Harvesting: An Exact Model and TDDFT Study
- PMID: 31457543
- PMCID: PMC6641107
- DOI: 10.1021/acsomega.7b00278
Singlet and Triplet Excited State Energy Ordering in Cyclopenta-Fused Polycyclic Aromatic Hydrocarbons (CP-PAHs) Suitable for Energy Harvesting: An Exact Model and TDDFT Study
Abstract
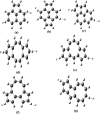
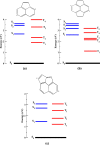
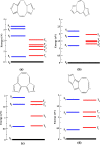
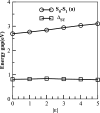
We calculated the ground and low-lying excited states of cyclopenta-fused polycyclic aromatic hydrocarbons (CP-PAHs) using exact diagonalization in full configuration interaction (CI) within the model Pariser-Parr-Pople Hamiltonian as well as a time-dependent density functional theory technique. The CP-PAHs include acenapthylene, isomers of pyracylene, cycloocta-pentalene, and three isomers of dicyclo-pentacyclo-octenes (DCPCO). We used the inherent symmetries of these systems to calculate the energy ordering of the lowest singlet (S1) and lowest triplet excited (T1) states with respect to the ground state (S0). The calculation shows that the lowest dipole allowed singlet absorption varies from 0.43 to 1.42 eV for most of these systems. Such an optical absorption range is very promising in harvesting solar radiation ranging from the visible to near-infrared region improving the efficiency of photovoltaic device application. The calculated optical gaps for pyracylene, acenapthylene, and two isomers of DCPCO are in very good agreement with experimental results reported in the literature. The calculated S1-T1 energy gaps (ΔST) in cycloocta-pentalene and in the DCPCO isomers are very small ranging from 0.01 to 0.2 eV, which is highly desirable in improving their electroluminescence efficiency in light-emitting device applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Scharber M. C.; Muhlbacher D.; Koppe M.; Denk P.; Waldauf V.; Heeger A. J.; Brabec C. J. Design Rules for Donors in Bulk-Heterojunction Solar Cells-Towards 10% Energy-Coversion Efficiency. Adv. Mater. 2006, 18, 789–794. 10.1002/adma.200501717. - DOI
-
- Veldman D.; Meskers S. C. J.; Janssen R. A. J. The Energy of Charge-Transfer States in Electron Donor-Acceptor Blends: Insight into the Energy Losses in Organic Solar Cells. Adv. Funct. Mater. 2009, 19, 1939–1948. 10.1002/adfm.200900090. - DOI
-
- Tandon K.; Ramasesha S.; Mazumdar S. Electron Correlation Effects in Electron-hole Recombination in Organic Light-emitting Diodes. Phys. Rev. B 2003, 67, 045109 10.1103/PhysRevB.67.045109. - DOI
-
- Kasha M. Characterization of Electronic Transitions in Complex Molecules. Discuss. Faraday Soc. 1950, 9, 14–19. 10.1039/df9500900014. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

