Exfoliated MoS2 and MoSe2 Nanosheets by a Supercritical Fluid Process for a Hybrid Mg-Li-Ion Battery
- PMID: 31457585
- PMCID: PMC6640930
- DOI: 10.1021/acsomega.7b00379
Exfoliated MoS2 and MoSe2 Nanosheets by a Supercritical Fluid Process for a Hybrid Mg-Li-Ion Battery
Abstract
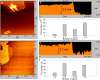
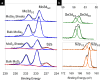
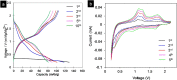
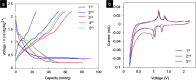
The ultrathin two-dimensional nanosheets of layered transition-metal dichalcogenides (TMDs) have attracted great interest as an important class of materials for fundamental research and technological applications. Solution-phase processes are highly desirable to produce a large amount of TMD nanosheets for applications in energy conversion and energy storage such as catalysis, electronics, rechargeable batteries, and capacitors. Here, we report a rapid exfoliation by supercritical fluid processing for the production of MoS2 and MoSe2 nanosheets. Atomic-resolution high-angle annular dark-field imaging reveals high-quality exfoliated MoS2 and MoSe2 nanosheets with hexagonal structures, which retain their 2H stacking sequence. The obtained nanosheets were tested for their electrochemical performance in a hybrid Mg-Li-ion battery as a proof of functionality. The MoS2 and MoSe2 nanosheets exhibited the specific capacities of 81 and 55 mA h g-1, respectively, at a current rate of 20 mA g-1.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Georgiou T.; Jalil R.; Belle B. D.; Britnell L.; Gorbachev R. V.; Morozov S. V.; Kim Y.-J.; Gholinia A.; Haigh S. J.; Makarovsky O.; et al. Vertical field-effect transistor based on graphene–WS2 heterostructures for flexible and transparent electronics. Nat. Nanotechnol. 2013, 8, 100–103. 10.1038/nnano.2012.224. - DOI - PubMed
LinkOut - more resources
Full Text Sources

