N-Geminal P/Al Lewis Pair-Alkyne Dipolar Cycloaddition to the Zwitterionic C2PNAl-Heterocyclopentene
- PMID: 31457589
- PMCID: PMC6682165
- DOI: 10.1021/acsomega.7b00236
N-Geminal P/Al Lewis Pair-Alkyne Dipolar Cycloaddition to the Zwitterionic C2PNAl-Heterocyclopentene
Abstract
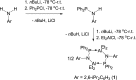
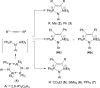
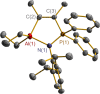
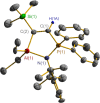
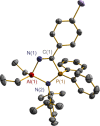
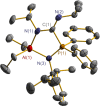
The N-geminal P/Al Lewis pair [Ph2PN(2,6-iPr2C6H3)AlEt2]2 (1) has been prepared and studied for reaction with a series of alkynes. The reaction of 1 with RC≡CR yielded zwitterionic C2PNAl-heterocyclopentene [Ph2 ⌜PN(2,6-iPr2C6H3)AlEt2](CR=⌝CR) (R = Me (2), Ph (3)); with PhC≡CEt produced two isomers, [Ph2 ⌜PN(2,6-iPr2C6H3)AlEt2](CPh=⌝CEt) (4a) and [Ph2 ⌜PN(2,6-iPr2C6H3)AlEt2](CEt=⌝CPh) (4b); and with other alkynes generated [Ph2 ⌜PN(2,6-iPr2C6H3)AlEt2](CR1=⌝CR2) (R1, R2 = CO2Et, Ph (5); SiMe3, Ph (6); PPh2, Ph (7); SiMe3,H (8); H, EtO (9)). Natural bond orbital analysis of the charge separation of the C≡C bond of alkynes was carried out, and then, the electronic matching interaction mode between the combined Lewis acid (AlEt2) and base (PPh2) groups of 1 and the C≡C bond of such alkynes was discussed. Reactions of 1 with alkene, nitrile, and carbodiimide molecules were also carried out, and cycloaddition compounds 10-12 were produced.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Stephan D. W.; Erker G. Angew. Chem., Int. Ed. 2010, 49, 46–76. 10.1002/anie.200903708. - DOI - PubMed
- Melen R. L. Chem. Commun. 2014, 50, 1161–1174. 10.1039/C3CC48036D. - DOI - PubMed
- Stephan D. W.; Erker G. Angew. Chem., Int. Ed. 2015, 54, 6400–6441. 10.1002/anie.201409800. - DOI - PubMed
- Stephan D. W. J. Am. Chem. Soc. 2015, 137, 10018–10032. 10.1021/jacs.5b06794. - DOI - PubMed
-
-
See selected articles
- Dureen M. A.; Stephan D. W. J. Am. Chem. Soc. 2009, 131, 8396–8397. 10.1021/ja903650w. - DOI - PubMed
- Dureen M. A.; Brown C. C.; Stephan D. W. Organometallics 2010, 29, 6594–6607. 10.1021/om1009044. - DOI
- Liedtke R.; Froehlich R.; Kehr G.; Erker G. Organometallics 2011, 30, 5222–5232. 10.1021/om200615n. - DOI
- Rosorius C.; Daniliuc C. G.; Froehlich R.; Kehr G.; Erker G. J. Organomet. Chem. 2013, 744, 149–155. 10.1016/j.jorganchem.2013.06.005. - DOI
- Liedtke R.; Scheidt F.; Ren J.; Schirmer B.; Cardenas A. J. P.; Daniliuc C. G.; Eckert H.; Warren T. H.; Grimme S.; Kehr G.; Erker G. J. Am. Chem. Soc. 2014, 136, 9014–9027. 10.1021/ja5028293. - DOI - PubMed
- Eller C.; Bussmann K.; Kehr G.; Wibbeling B.; Daniliuc C. G.; Erker G. Chem. Commun. 2014, 50, 1980–1982. 10.1039/c3cc47769j. - DOI - PubMed
-
-
-
See selected articles
- Kolb H. C.; Finn M. G.; Sharpless K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. - DOI - PubMed
- Angell Y. L.; Burgess K. Chem. Soc. Rev. 2007, 36, 1674–1689. 10.1039/b701444a. - DOI - PubMed
- Díez-González S.; Nolan S. P. Angew. Chem., Int. Ed. 2008, 47, 8881–8884. 10.1002/anie.200803289. - DOI - PubMed
- Liang L.; Astruc D. Coord. Chem. Rev. 2011, 255, 2933–2945. 10.1016/j.ccr.2011.06.028. - DOI
-
-
- Freitag S.; Krebs K. M.; Henning J.; Hirdler J.; Schubert H.; Wesemann L. Organometallics 2013, 32, 6785–6791. 10.1021/om400736e. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

