Bite-Angle-Regulated Coordination Geometries: Tetrahedral and Trigonal Bipyramidal in Ni(II) with Biphenyl-Appended (2-Pyridyl)alkylamine N, N'-Bidentate Ligands
- PMID: 31457593
- PMCID: PMC6640959
- DOI: 10.1021/acsomega.7b00119
Bite-Angle-Regulated Coordination Geometries: Tetrahedral and Trigonal Bipyramidal in Ni(II) with Biphenyl-Appended (2-Pyridyl)alkylamine N, N'-Bidentate Ligands
Abstract
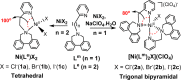
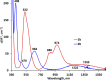
Two simple biphenyl-appended (2-pyridyl)alkylamine N-bidentate ligands, Le and Lm, having ethylene and methylene spacers between donor groups, with bite angles Le ≈ 100° and Lm ≈ 80°, dictate pseudotetrahedral and trigonal-bipyramidal geometries in six high-spin Ni(II)-halide complexes, [Ni(Le)X2] and [Ni(Lm)2X](ClO4) (where X = Cl-, Br-, I-), respectively. The structures in the solid state, determined using X-ray crystallography, and in solution, determined using spectroscopic methods (UV-vis-NIR and paramagnetic 1H NMR), which complement each other, are described.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Stack T. D. P. Complexity with Simplicity: a Steric continuum of Chelating Diamines with Copper(I) and Dioxygen. Dalton Trans. 2003, 1881–1889. 10.1039/B300201M. - DOI
- Holland P. L. Electronic Structure and Reactivity of Three Coordinate Iron Complexes. Acc. Chem. Res. 2008, 41, 905–914. 10.1021/ar700267b. - DOI - PMC - PubMed
- Tonzetich Z. J.; Heroguel F.; Do L.; Lippard S. J. Chemistry of Nitrosyliron Complexes Supported by a β-Diketiminate Ligand. Inorg. Chem. 2011, 50, 1570–1579. 10.1021/ic102300d. - DOI - PMC - PubMed
- Taki M.; Teramae S.; Nagatomo S.; Tachi Y.; Kitagawa T.; Itoh S.; Fukuzumi S. Fine-Tuning of Copper(I)-Dioxygen Reactivity by 2-(2-Pyridyl)ethylamine Bidentate Ligands. J. Am. Chem. Soc. 2002, 124, 6367–6377. 10.1021/ja026047x. - DOI - PubMed
- Aboelella N. W.; Lewis E. A.; Reynolds A. M.; Brennessel W. W.; Cramer C. J.; Tolman W. B. Snapshots of Dioxygen Activation by Copper: The Structure of a 1:1 Cu/O2 Adduct and Its Use in Syntheses of Asymmetric Bis(μ-oxo) Complexes. J. Am. Chem. Soc. 2002, 124, 10660–10661. 10.1021/ja027164v. - DOI - PubMed
- Benson E. E.; Rheingold A. L.; Kubiak C. P. Synthesis and Characterization of 6,6′-(2,4,6-Triisopropylphenyl)-2,2′-bipyridine(tripbipy) and Its Complexes of the Late First Row Transition Metals. Inorg. Chem. 2010, 49, 1458–1464. 10.1021/ic9016382. - DOI - PubMed
-
- Yao S.; Driess M. Lessons from Isolable Nickel(I) Precursor Complexes for Small Molecule Activation. Acc. Chem. Res. 2012, 45, 276–287. 10.1021/ar200156r. - DOI - PubMed
- Wiese S.; Kapoor P.; Williams K. D.; Warren T. H. Nitric Oxide Oxidatively Nitrosylates Ni(I) and Cu(I) C-Organonitroso Adducts. J. Am. Chem. Soc. 2009, 131, 18105–18111. 10.1021/ja903550n. - DOI - PubMed
- Garcia-Bosch I.; Ribas X.; Costas M. Well-Defined Heterometallic and Unsymmetric M2O2 Complexes Arising from Binding and Activation of O2. Eur. J. Inorg. Chem. 2012, 2012, 179–187. 10.1002/ejic.201100957. - DOI
- Rhinehart J. L.; Brown L. A.; Long B. K. A Robust Ni(II) α-Diimine Catalyst for High Temperature Ethylene Polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. 10.1021/ja408905t. - DOI - PubMed
-
- Corona T.; Company A. Spectroscopically Characterized Synthetic Mononuclear Nickel–Oxygen Species. Chem. Eur. J. 2016, 22, 13422–13429. 10.1002/chem.201602414. - DOI - PubMed
- Yao S.; Bill E.; Milsmann C.; Wieghardt K.; Driess M. A “Side-on” Superoxonickel Complex [LNi(O2)] with a Square-Planar Tetracoordinate Nickel(II) Center and Its Conversion into[LNi(m-OH)2 NiL]. Angew. Chem., Int. Ed. 2008, 47, 7110–7113. 10.1002/anie.200802234. - DOI - PubMed
-
- Johnson L. K.; Killian C. M.; Brookhart M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. 10.1021/ja00128a054. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

