Solvent-Controlled, Tunable Domino Reaction of 3-Ylideneoxindoles with in Situ-Generated α-Aryldiazomethanes: A Facile Access to 3-Spirocyclopropyl-2-oxindole and Pyrazoloquinazolinone Scaffolds
- PMID: 31457971
- PMCID: PMC6644904
- DOI: 10.1021/acsomega.8b01857
Solvent-Controlled, Tunable Domino Reaction of 3-Ylideneoxindoles with in Situ-Generated α-Aryldiazomethanes: A Facile Access to 3-Spirocyclopropyl-2-oxindole and Pyrazoloquinazolinone Scaffolds
Abstract
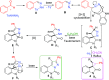
A mild and efficient solvent-controlled, metal-free switchable 1,3-dipolar cycloaddition/ring contraction or ring expansion domino reaction of 3-ylideneoxindoles with in situ-generated α-aryldiazomethanes has been developed. This domino reaction provided a series of aryl-substituted 3-spirocyclopropyl-2-oxindoles and pyrazoloquinazolinones with excellent regio- and diastereoselectivity from common substrates under varying solvent conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Nicolaou K. C.; Vourloumis D.; Winssinger N.; Baran P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem., Int. Ed. 2000, 39, 44.10.1002/(sici)1521-3773(20000103)39:1a3.3.co;2-c. - DOI - PubMed
- Noyori R. Asymmetric synthesis via axially dissymmetric molecules. A binaphthol-modified complex aluminum hydride reagent possessing extremely high ability of chiral recognition. Chem. Commun. 2005, 1807.10.1039/b502713f. - DOI
- Nicolaou K. C.; Synder S. A. The essence of total synthesis. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 11929.10.1073/pnas.0403799101. - DOI - PMC - PubMed
- Greshock T. J.; Williams R. M. Improved biomimetic total synthesis of d,l-stephacidin A. Org. Lett. 2007, 9, 4255.10.1021/ol701845t. - DOI - PubMed
- Lin H.; Danishefsky S. J. A Suggestive Link between the Chemistry and Biology of Epothilones. Angew. Chem., Int. Ed. 2003, 42, 36.10.1002/anie.200390048. - DOI - PubMed
-
-
For reviews of spirocyclicoxindoles, see:
- Singh G. S.; Desta Z. Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104.10.1021/cr300135y. - DOI - PubMed
- Trost B.; Brennan M. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis 2009, 2009, 3003.10.1055/s-0029-1216975. - DOI
- Galliford C. V.; Scheidt K. A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem., Int. Ed. 2007, 46, 8748.10.1002/anie.200701342. - DOI - PubMed
-
-
- Tan B.; Candeias N. R.; Barbas C. F. III Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst. Nat. Chem. 2011, 3, 473.10.1038/nchem.1039. - DOI - PubMed
- Peng J.; Huang X.; Jiang L.; Cui H. L.; Chen Y. C. Tertiary amine-catalyzed chemoselective and asymmetric [3 + 2] annulation of morita–baylis–hillman carbonates of isatins with propargyl sulfones. Org. Lett. 2011, 13, 4584.10.1021/ol201776h. - DOI - PubMed
- Bergonzini G.; Melchiorre P. Dioxindole in Asymmetric Catalytic Synthesis: Routes to Enantioenriched 3-Substituted 3-Hydroxyoxindoles and the Preparation of Maremycin A. Angew. Chem., Int. Ed. 2012, 51, 971.10.1002/anie.201107443. - DOI - PubMed
- Cheng D. J.; Ishihara Y.; Tan B.; Barbas C. F. III. Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catal. 2014, 4, 743.10.1021/cs401172r. - DOI
- Trost B. M.; Bringley D. A.; Zhang T.; Cramer N. Rapid access to spirocyclic oxindole alkaloids: application of the asymmetric palladium-catalyzed [3 + 2] trimethylenemethane cycloaddition. J. Am. Chem. Soc. 2013, 135, 16720.10.1021/ja409013m. - DOI - PMC - PubMed
- Yeung B. K. S.; Zou B.; Rottmann M.; Lakshminarayana S. B.; Ang S. H.; Leong S. Y.; Tan J.; Wong J.; Keller-Maerki S.; Fischli C.; Goh A.; Schmitt E. K.; Krastel P.; Francotte E.; Kuhen K.; Plouffe D.; Henson K.; Wagner T.; Winzeler E. A.; Petersen F.; Brun R.; Dartois V.; Diagana T. T.; Keller T. H. Spirotetrahydro β-carbolines (spiroindolones): A new class of potent and orally efficacious compounds for the treatment of malaria. J. Med. Chem. 2010, 53, 5155.10.1021/jm100410f. - DOI - PMC - PubMed
- Lee B. H. Synthesis and Modification of Marcfortine and Paraherquamide Class of Anthelmintics. Stud. Nat. Prod. Chem. 2003, 28, 331.and references therein10.1016/s1572-5995(03)80145-x. - DOI
- Jiang X.; Cao Y.; Wang Y.; Liu L.; Shen F.; Wang R. A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J. Am. Chem. Soc. 2010, 132, 15328.10.1021/ja106349m. - DOI - PubMed
- Zheng Y.; Tice C. M.; Singh S. B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673.10.1016/j.bmcl.2014.06.081. - DOI - PubMed
- Ye N.; Chen H. Y.; Wold E. A.; Shi P.-Y.; Zhou J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016, 2, 382.10.1021/acsinfecdis.6b00041. - DOI - PMC - PubMed
-
- Schwartz R. E.; Hirsch C. F.; Springer J. P.; Pettibone D. J.; Zink D. L. Unusual cyclopropane-containing hapalindolinones from a cultured cyanobacterium. J. Org. Chem. 1987, 52, 3704.10.1021/jo00392a045. - DOI
- Sampson P. B.; Liu Y.; Li S.-W.; Forrest B. T.; Pauls H. W.; Edwards L. G.; Feher M.; Patel N. K. B.; Laufer R.; Pan G.. The Discovery of Polo-like Kinase 4 Inhibitors: Design and Optimization of Spiro[cyclopropane-1,3′[3H]indol]-2′(1′H).ones as Orally Bioavailable Antitumor Agents. USWO/2010/115279A12010.
- Pauls H. W.; Li S.-W.; Sampson P. B.; Forrest B. T.. Plk-4 Inhibitors and Method of Treating Cancer with Same. USWO/2012/048411A12012.
- Chen L.; Feng L.; He Y.; Huang M.; Yun H.. Spiro Indole – Cyclopropane Indolinones Useful as AMPK Modulators. USWO/2011/070039A12011.
-
- Ellis D.; Kuhen K. L.; Anaclerio B.; Wu B.; Wolff K.; Yin H.; Bursulaya B.; Caldwell J.; Karanewsky D.; He Y. Design, synthesis, and biological evaluations of novel quinolones as HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 4246.10.1016/j.bmcl.2006.05.073. - DOI - PubMed
- Jiang T.; Kuhen K. L.; Wolff K.; Yin H.; Bieza K.; Caldwell J.; Bursulaya B.; Wu T. Y.; He Y. Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I. Bioorg. Med. Chem. Lett. 2006, 16, 2105.10.1016/j.bmcl.2006.01.073. - DOI - PubMed
- He Y.; Jiang T.; Kuhen K. L.; Ellis D. A.; Wu B.; Wu T. Y.; Bursulaya B.. Oxindoles with Anti-HIV Activity. US7,205,328B22007.
LinkOut - more resources
Full Text Sources