Room-Temperature, Copper-Free Sonogashira Reactions Facilitated by Air-Stable, Monoligated Precatalyst [DTBNpP] Pd(crotyl)Cl
- PMID: 31458021
- PMCID: PMC6644404
- DOI: 10.1021/acsomega.8b01868
Room-Temperature, Copper-Free Sonogashira Reactions Facilitated by Air-Stable, Monoligated Precatalyst [DTBNpP] Pd(crotyl)Cl
Abstract
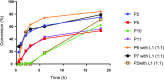
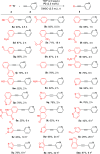
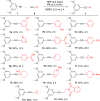
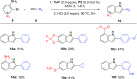
A novel application of [DTBNpP] Pd(crotyl)Cl (DTBNpP = di-tert-butylneopentylphosphine) (P2), an air-stable, commercially available palladium precatalyst that allows rapid access to a monoligated state, has been identified for room-temperature, copper-free Sonogashira couplings of challenging aryl bromides and alkynes. The mild reaction conditions with TMP in dimethyl sulfoxide afford up to 97% yields, excellent functional group tolerability, and broad reaction compatibility with access to one-pot indole formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Jenny N. M.; Mayor M.; Eaton T. R. Phenyl-Acetylene Bond Assembly: A Powerful Tool for the Construction of Nanoscale Architectures. Eur. J. Org. Chem. 2011, 2011, 4965–4983. 10.1002/ejoc.201100176. - DOI
-
- Wang D.; Gao S. Sonogashira coupling in natural product synthesis. Org. Chem. Front. 2014, 1, 556.10.1039/C3QO00086A. - DOI
-
- Sonogashira K.; Tohda Y.; Hagihara N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. 10.1016/S0040-4039(00)91094-3. - DOI
LinkOut - more resources
Full Text Sources

