Revealing How Alkali Cations Affect the Surface Reactivity of Stainless Steel in Alkaline Aqueous Environments
- PMID: 31458146
- PMCID: PMC6644133
- DOI: 10.1021/acsomega.8b02227
Revealing How Alkali Cations Affect the Surface Reactivity of Stainless Steel in Alkaline Aqueous Environments
Abstract
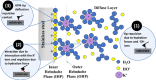
Stainless steel is a ubiquitous structural material and one that finds extensive use in core-internal components in nuclear power plants. Stainless steel features superior corrosion resistance (e.g., as compared to ordinary steel) due to the formation of passivating iron and/or chromium oxides on its surfaces. However, the breakdown of such passivating oxide films, e.g., due to localized deformation and slip line formation following exposure to radiation, or aggressive ions renders stainless steel susceptible to corrosion-related degradation. Herein, the effects of alkali cations (i.e., K+, Li+) and the interactions between the passivated steel surface and the solution are examined using 304L stainless steel. Scanning electrochemical microscopy and atomic force microscopy are used to examine the inert-to-reactive transition of the steel surface both in the native state and in the presence of applied potentials. Careful analysis of interaction forces, in solution, within ≤10 nm of the steel surface, reveals that the interaction between the hydrated alkali cations and the substrate affects the structure of the electrical double layer (EDL). As a result, a higher surface reactivity is indicated in the presence of Li+ relative to K+ due to the effects of the former species in disrupting the EDL. These findings provide new insights into the role of the water chemistry not only on affecting metallic corrosion but also in other applications, such as batteries and electrochemical devices.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Bruemmer S. M.; Was G. S. Microstructural and Microchemical Mechanisms Controlling Intergranular Stress Corrosion Cracking in Light-Water-Reactor Systems. J. Nucl. Mater. 1994, 216, 348–363. 10.1016/0022-3115(94)90020-5. - DOI
-
- Nishioka H.; Fukuya K.; Fujji K.; Kitsunai Y. Deformation Structure in Highly Irradiated Stainless Steels. J. Nucl. Sci. Technol. 2008, 45, 274–287. 10.1080/18811248.2008.9711437. - DOI
-
- McMurtrey M. D.; Was G. S.; Cui B.; Robertson I.; Smith L.; Farkas D. Strain Localization at Dislocation Channel–Grain Boundary Intersections in Irradiated Stainless Steel. Int. J. Plast. 2014, 56, 219–231. 10.1016/j.ijplas.2014.01.001. - DOI
-
- Carvajal-Ortiz R. A.; Plugatyr A.; Svishchev I. M. On the pH Control at Supercritical Water-Cooled Reactor Operating Conditions. Nucl. Eng. Des. 2012, 248, 340–342. 10.1016/j.nucengdes.2012.03.038. - DOI
-
- Sato N. A Theory for Breakdown of Anodic Oxide Films on Metals. Electrochim. Acta 1971, 16, 1683–1692. 10.1016/0013-4686(71)85079-X. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

