Versatile Synthetic Approach for Selective Diversification of Bicyclic Aza-Diketopiperazines
- PMID: 31458181
- PMCID: PMC6643515
- DOI: 10.1021/acsomega.8b01752
Versatile Synthetic Approach for Selective Diversification of Bicyclic Aza-Diketopiperazines
Abstract
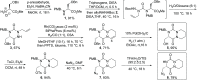
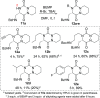
Herein, we report a convenient synthesis of unprecedented aza-diketopiperazines (aza-DKPs). The strategy is based on selective diversification of bicyclic aza-DKP scaffolds by click reaction, N-acylation, and/or N-alkylation. These scaffolds containing either azido or amino groups were obtained by a key Rh(I)-catalyzed hydroformylative cyclohydrocarbonylation reaction of allyl-substituted aza-DKP. The methodology is readily amenable to the parallel synthesis of original aza-DKPs to enlarge the chemical diversity of aza-heterocycles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Hoffman R. V.; Nayyar N. Reaction of hydrazines with.alpha.-lactams for the preparation of 1,2,4-triazine-3,6-diones and aza-urea peptide mimetics. J. Org. Chem. 1995, 60, 5992–5994. 10.1021/jo00123a048. - DOI
- Hoffman R. V.; Reddy M. M.; Klumas C. M.; Cervantes-Lee F. The reactions of hydrazines with α-lactams. Regiochemistry of hydrazine addition and subsequent ring closure to N-aminohydantoins or 1,2,4-triazine-3,6-diones. J. Org. Chem. 1998, 63, 9128–9130. 10.1021/jo981650c. - DOI
-
- Obreza A.; Urleb U. A two-step synthesis of hexahydropyrrolo-[1,2-d][1,2,4]triazine-1,4-dione and related compounds. Synth. Commun. 2003, 33, 1011–1018. 10.1081/SCC-120016366. - DOI
LinkOut - more resources
Full Text Sources

