Terbium Oxalatophosphonate as Efficient Multiresponsive Luminescent Sensors for Chromate Anions and Tryptophan Molecules
- PMID: 31458303
- PMCID: PMC6643623
- DOI: 10.1021/acsomega.8b02486
Terbium Oxalatophosphonate as Efficient Multiresponsive Luminescent Sensors for Chromate Anions and Tryptophan Molecules
Abstract
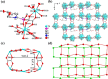
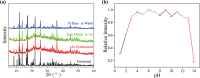
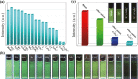
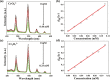
A stable 2D terbium oxalatophosphonate with green luminescence, namely, [Tb2(H3L)(C2O4)3(H2O)4]·2H2O (1), has been hydrothermally obtained by using (4-carboxypiperidyl)-N-methylenephosphonic acid (H3L) and oxalate ligand. The luminescent investigation indicates that the emission behavior of compound 1 shows high water and pH stabilities. It can be applied as a multiresponsive luminescent probe with high selectivity, high sensitivity, recycling capability, and fast sensing of CrO4 2-, Cr2O7 2- anions and tryptophan (Trp) molecules in aqueous solution through the luminescence quenching effect. Moreover, the sensing results can be distinguished by the naked eye under the irradiation of UV light of 254 nm. In addition, the probable mechanisms for the quenching behavior are also discussed, which can be mainly attributed to the competitive absorption of excitation energy between compound 1 and the analytes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

