Chiral Discrimination in Rhodium(I) Catalysis by 2,5-Disubstituted 1,3 a,4,6 a-Tetrahydropenatalene Ligands-More Than Just a Twist of the Olefins?
- PMID: 31458613
- PMCID: PMC6641409
- DOI: 10.1021/acsomega.8b00127
Chiral Discrimination in Rhodium(I) Catalysis by 2,5-Disubstituted 1,3 a,4,6 a-Tetrahydropenatalene Ligands-More Than Just a Twist of the Olefins?
Abstract
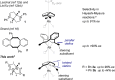
Chiral dienes are useful ligands in a number of asymmetric transition-metal-catalyzed reactions. Here, we evaluate the efficiency of 2,5-disubstituted 1,3a,4,6a-tetrahydropentalenes as ligands to rhodium(I). 2,5-Dibenzyl and diphenyl tetrahydropentalenes were synthesized in two steps and resolved, either chromatographically, or through fractional crystallization of diastereomeric rhodium(I) salts. When evaluated in a 1,4-arylation reaction, the 2,5-dibenzyl ligand gave up to 99% ee. The use of a well-defined rhodium complex as catalyst, Cs2CO3 as the base, and toluene/water as solvent was found to have a pronounced beneficial effect on the selectivity of the reaction. The homologous 2,5-diphenyl ligand on the other hand proved to be highly prone to racemization/loss of chirality during catalysis. Control experiments reveal that this rearrangement proceeds via a rhodium-mediated 1,3-hydride shift. Implications for ligand design and catalysis are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



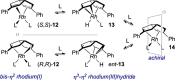
References
-
-
For pioneering studies on dienes as steering ligands in asymmetric catalysis, see:
- Hayashi T.; Ueyana K.; Tokunaga N.; Yoshida K. A Chiral Chelating Diene as a New Type of Chiral Ligand for Transition Metal Catalysts: Its Preparation and Use for the Rhodium-Catalyzed Asymmetric 1,4-Addition. J. Am. Chem. Soc. 2003, 125, 11508–11509. 10.1021/ja037367z. - DOI - PubMed
- Fischer C.; Defieber C.; Suzuki T.; Carriera E. M. Readily Available [2.2.2]-Bicyclooctadienes as New Chiral Ligands for Ir(I): Catalytic, Kinetic Resolution of Allyl Carbonates. J. Am. Chem. Soc. 2004, 126, 1628–1629. 10.1021/ja0390707. - DOI - PubMed
-
-
-
For reviews, see:
- Glorius F. Chiral Olefin Ligands—New “Spectators” in Asymmetric Catalysis. Angew. Chem., Int. Ed. 2004, 43, 3364–3366. 10.1002/anie.200301752. - DOI - PubMed
- Defieber C.; Grützmacher H.; Carriera E. M. Chiral Olefins as Steering Ligands in Asymmetric Catalysis. Angew. Chem., Int. Ed. 2008, 47, 4482–4502. 10.1002/anie.200703612. - DOI - PubMed
- Johnson J. B.; Rovis T. More than Bystanders: The Effect of Olefins on Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew. Chem., Int. Ed. 2008, 47, 840–871. 10.1002/anie.200700278. - DOI - PubMed
- Shintani R.; Hayashi T. Chiral Diene Ligands for Asymmetric Catalysis. Aldrichimica Acta 2009, 42, 31–38.
- Feng C.-G.; Xu M.-H.; Lin G.-Q. Development of Bicyclo[3.3.0]octadiene- or Dicyclopentadiene-Based Chiral Diene Ligands for Transition-Metal-Catalyzed Reactions. ChemInform 2011, 42, 1345–1356. 10.1002/chin.201146240. - DOI
-
-
-
For selected recent examples, see:
- Wang Z.-Q.; Feng C.-G.; Zhang S.-S.; Xu M.-H.; Lin G-.Q. Rhodium-Catalyzed Asymmetric Conjugate Addition of Organoboronic Acids to Nitroalkenes Using Chiral Bicyclo[3.3.0] Diene Ligands. Angew. Chem., Int. Ed. 2010, 49, 5780–5783. 10.1002/anie.201001883. - DOI - PubMed
- Chen C. C.; Gopula B.; Syu J.-F.; Pan J.-H.; Kuo T. S.; Wu P.-Y.; Henschke J. P.; Wu H.-L. Enantioselective and Rapid Rh-Catalyzed Arylation of N-Tosyl- and N-Nosylaldimines in Methanol. J. Org. Chem. 2014, 79, 8077–8085. 10.1021/jo5012653. - DOI - PubMed
- Zhang Y.-F.; Chen D.; Chen W.-W.; Xu M. H. Construction of Cyclic Sulfamidates Bearing Two gem-Diaryl Stereocenters through a Rhodium-Catalyzed Stepwise Asymmetric Arylation Protocol. Org. Lett. 2016, 18, 2726–2729. 10.1021/acs.orglett.6b01183. - DOI - PubMed
- Bürki C.; Whyte A.; Arndt S.; Hashmi A. S. K.; Lautens M. Expanding the Scope of the Gold(I)-Catalyzed Rautenstrauch Rearrangement: Protic Additives. Org. Lett. 2016, 18, 5058–5061. 10.1021/acs.orglett.6b02505. - DOI - PubMed
-
-
-
For selected recent examples, see:
- Zhang Q.; Stockdale D. P.; Mixdorf J. C.; Topczewski J. J.; Nguyen H. M. Iridium-Catalyzed Enantioselective Fluorination of Racemic, Secondary Allylic Trichloroacetimidates. J. Am. Chem. Soc. 2015, 137, 11912–11915. 10.1021/jacs.5b07492. - DOI - PubMed
- Nagamoto M.; Yanagi T.; Nishimura T.; Yorimitsu H. Asymmetric Cyclization of N-Sulfonyl Alkenyl Amides Catalyzed by Iridium/Chiral Diene Complexes. Org. Lett. 2016, 18, 4474–4477. 10.1021/acs.orglett.6b01954. - DOI - PubMed
-
-
- Grundl M. A.; Kennedy-Smith J.; Trauner D. Rational Design of a Chiral Palladium(0) Olefin Complex of Unprecedented Stability. Organometallics 2005, 24, 2831–2833. 10.1021/om050277d. - DOI
- Zhang S.-S.; Wang Z.-Q.; Xu M.-H.; Lin G.-Q. Chiral Diene as the Ligand for the Synthesis of Axially Chiral Compounds via Palladium-Catalyzed Suzuki–Miyaura Coupling Reaction. Org. Lett. 2010, 12, 5546–5549. 10.1021/ol102521q. - DOI - PubMed
LinkOut - more resources
Full Text Sources

