Synthesis of Highly Efficient Bifunctional Ag/Co3O4 Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Medium
- PMID: 31458922
- PMCID: PMC6644694
- DOI: 10.1021/acsomega.8b00799
Synthesis of Highly Efficient Bifunctional Ag/Co3O4 Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Medium
Abstract
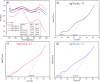
Ag/Co3O4 catalysts using three different modes of solution combustion synthesis were developed and characterized by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy to identify crystallite size, oxidation state, composition, and morphology. Cyclic voltammetry and linear sweep voltammetry measurements for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) confirm the bifunctionality of the electrocatalysts. The electrochemical evaluation indicates that a synergic effect between Ag and Co enhances the activity through the fast breaking of O-O bond in the molecular oxygen to enhance the reduction mechanism. The high content of cobalt (Co) in the catalyst Ag/Co3O4-12, synthesized by second wave combustion, improves the activity for ORR, and the reaction mechanism follows a 3.9 number of electron transfer in overall reaction. The kinetic and limiting current densities of Ag/Co3O4-12 are maximum when compared to those of other Ag/Co3O4 catalysts and are very close to commercial Pt/C. Moreover, the maximum current density of OER for Ag/Co3O4-12 makes it a promising candidate for various bifunctional electrocatalytic applications such as fuel cells and metal-air batteries.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Rabis A.; Rodriguez P.; Schmidt T. J. Electrocatalysis for polymer electrolyte fuel cells: recent achievements and future challenges. ACS Catal. 2012, 2, 864–890. 10.1021/cs3000864. - DOI
-
- Cao R.; Lee J.-S.; Liu M.; Cho J. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. 10.1002/aenm.201200013. - DOI
-
- Zhang Z.; Liu J.; Gu J.; Su L.; Cheng L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. 10.1039/c3ee43886d. - DOI
-
- Jiang S.; Song S.; Du Y.; Zhai C.; Zhu M. Constructing highly stretchable and superstable electrode with N-doped double-walled carbon nanotubes/poly(m-phenylene isophthalamide) for oxygen reduction reaction. Chem. Eng. J. 2017, 327, 1077–1084. 10.1016/j.cej.2017.07.014. - DOI
-
- Zheng F.; Yin Z.; Xia H.; Bai G.; Zhang Y. Porous MnO@C nanocomposite derived from metal-organic frameworks as anode materials for long-life lithium-ion batteries. Chem. Eng. J. 2017, 327, 474–480. 10.1016/j.cej.2017.06.097. - DOI
LinkOut - more resources
Full Text Sources

