Hybrids Based on Graphene Oxide and Porphyrin as Tools for Detection and Stabilization of DNA G-Quadruplexes
- PMID: 31459228
- PMCID: PMC6645567
- DOI: 10.1021/acsomega.8b01366
Hybrids Based on Graphene Oxide and Porphyrin as Tools for Detection and Stabilization of DNA G-Quadruplexes
Abstract
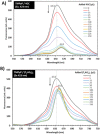
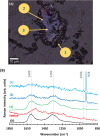
Telomerase inhibition has been an important strategy in cancer therapies, but for which effective drugs are still required. Here, noncovalent hybrid nanoplatforms containing the tetracationic 5,10,15,20-tetrakis(1-methyl-pyridinium-4-yl)porphyrin (TMPyP) and graphene oxide (GO) were prepared for promoting telomerase inhibition through the selective detection and stabilization of DNA guanine-quadruplex (G-Q) structures. Upon binding TMPyP to the GO sheets, the typical absorption bands of porphyrin have been red-shifted and the fluorescence emission was quenched. Raman mapping was used for the first time to provide new insights into the role of the electrostatic and π-π stacking interactions in the formation of such hybrids. The selective recovery of fluorescence observed during the titration of TMPyP@GO with G-Q, resembles a selective "turn-off-on" fluorescence sensor for the detection of G-Q, paving the way for a new class of antitumor drugs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

