Diastereoisomerism, Stability, and Morphology of Substituted meso-4-Sulfonatophenylporphyrin J-Aggregates
- PMID: 31459664
- PMCID: PMC6648571
- DOI: 10.1021/acsomega.8b03176
Diastereoisomerism, Stability, and Morphology of Substituted meso-4-Sulfonatophenylporphyrin J-Aggregates
Abstract
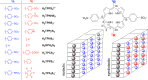
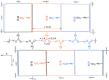
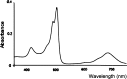
The comparison between nanoparticle morphologies of the J-aggregates of different meso-4-sulfonatophenylporphyrins showing non-sulfonato groups at some of the meso-positions constitutes an ultimate proof of the 2D crystal-like character of the basic self-assembly motif of this family of J-aggregates. Diastereoisomerism stemming from the tacticity of the relative configurations in relation to the J-aggregate bidimensional sheet is the key factor that determines both the striking monolayer in solution and also the hierarchical pathways leading to different nanoparticle morphologies upon further growth. The unexpected stability of such large monolayered sheets made up of porphyrin units is probably caused by the support originated at both surface faces by the double layer potentials of the peripheral ionic substituents. These double layer potentials play a driving role in the subsequent 3D growth of the monolayers, as deduced herein from the determining role of tacticity both in the stability of the J-aggregate sheet and in its evolution either to monolayered or to bilayered nanoparticles. The stabilizing role of the forces at the electrical double layer of the particle suggests a relationship between these forces and the previously reported detection of racemic biases when shear hydrodynamic forces are in action during the aggregation process.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Effect of Hydrodynamic Forces on meso-(4-Sulfonatophenyl)-Substituted Porphyrin J-Aggregate Nanoparticles: Elasticity, Plasticity and Breaking.Chemistry. 2016 Jul 4;22(28):9740-9. doi: 10.1002/chem.201600874. Epub 2016 May 30. Chemistry. 2016. PMID: 27238461
-
Hydrodynamic Effects in Soft-matter Self-assembly: The Case of J-Aggregates of Amphiphilic Porphyrins.Chem Rec. 2017 Jul;17(7):713-724. doi: 10.1002/tcr.201600133. Epub 2017 Jan 20. Chem Rec. 2017. PMID: 28105702 Review.
-
Cl- complexation induced H- and J-aggregation of meso-tetrakis(4-sulfonatothienyl)porphyrin diacid in aqueous solution.J Phys Chem B. 2011 Jun 23;115(24):7773-80. doi: 10.1021/jp2018428. Epub 2011 May 25. J Phys Chem B. 2011. PMID: 21568355
-
Kinetic control of the supramolecular chirality of porphyrin J-aggregates.Chemistry. 2012 Jul 9;18(28):8820-6. doi: 10.1002/chem.201200881. Epub 2012 Jun 8. Chemistry. 2012. PMID: 22678975
-
Hydrodynamic fragmentation of nanoparticle aggregates at orthokinetic coagulation.Adv Colloid Interface Sci. 2005 Jun 30;114-115:119-31. doi: 10.1016/j.cis.2004.07.012. Epub 2005 Feb 26. Adv Colloid Interface Sci. 2005. PMID: 15936286
Cited by
-
Tunable Supramolecular Chirogenesis in the Self-Assembling of Amphiphilic Porphyrin Triggered by Chiral Amines.Int J Mol Sci. 2020 Nov 13;21(22):8557. doi: 10.3390/ijms21228557. Int J Mol Sci. 2020. PMID: 33202819 Free PMC article.
-
The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update.Molecules. 2019 Nov 26;24(23):4307. doi: 10.3390/molecules24234307. Molecules. 2019. PMID: 31779097 Free PMC article. Review.
References
-
- Elemans J. A. A. W.; van Hameren R.; Nolte R. J. M.; Rowan A. E. Molecular Materials by Self-Assembly of Porphyrins, Phthalocyanines, and Perylenes. Adv. Mater. 2006, 18, 1251–1266. 10.1002/adma.200502498. - DOI
-
- D’Souza F.; Smith P. M.; Zandler M. E.; McCarty A. L.; Itou M.; Araki Y.; Ito O. Energy Transfer Followed by Electron Transfer in a Supramolecular Triad Composed of Boron Dipyrrin, Zinc Porphyrin, and Fullerene: A Model for the Photosynthetic Antenna-Reaction Center Complex. J. Am. Chem. Soc. 2004, 126, 7898–7907. 10.1021/ja030647u. - DOI - PubMed
