Serendipitous Formation of 2 H-Pyrazolo[3,4- d]pyridazin-7(6 H)-ones from 3-Arylsydnones
- PMID: 31459679
- PMCID: PMC6647958
- DOI: 10.1021/acsomega.8b02013
Serendipitous Formation of 2 H-Pyrazolo[3,4- d]pyridazin-7(6 H)-ones from 3-Arylsydnones
Abstract
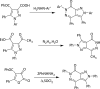
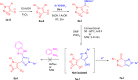
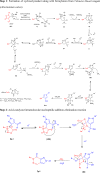
Fused nitrogen heterocyclesnamely, pyrazolo[3,4-d]pyridazin-7(6H)-ones have been obtained by exploiting the 1,3-dipolar nature of N-arylsydnones, from hydrazones of 3-aryl-4-acetylsydnones via the Vilsmeier-Haack strategy. Facile intramolecular nucleophilic addition followed by CO2 elimination under reflux or upon microwave irradiation was presented. Plausible mechanisms for the formation of the title compounds are proffered. Structure confirmatory evidence came from single-crystal X-ray crystallography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Stewart F. H. C. The Chemistry of Sydnones. Chem. Rev. 1964, 64, 129–147. 10.1021/cr60228a004. - DOI
-
- Clapp L. B.1,2,3 and 1,2,4-Oxadiazoles. In Comprehensive Heterocyclic Chemistry; Katritzky A. R., Rees C. W., Eds.; Pergamon Press: Oxford, U.K., 1984; Vol. 6, pp 365–378.
-
- Newton C. G.; Ramsden C. A. Meso-ionic heterocycles (1976-1980). Tetrahedron 1982, 38, 2965–3011. 10.1016/0040-4020(82)80186-5. - DOI
- Browne D. L.; Taylor J. B.; Plant A.; Harrity J. P. A. Cross coupling of bromo sydnones: Development of a flexible route toward functionalized pyrazoles. J. Org. Chem. 2009, 74, 396–400. 10.1021/jo802240e. - DOI - PubMed
- Brown A. W.; Harrity J. P. A. Direct arylation of sydnones with aryl chlorides toward highly substituted pyrazoles. J. Org. Chem. 2015, 80, 2467–2472. 10.1021/acs.joc.5b00143. - DOI - PubMed
- Favre C.; de Cremoux L.; Badaut J.; Friscourt F. Sydnone reporters for highly fluorogenic copper-free click ligations. J. Org. Chem. 2018, 83, 2058–2066. 10.1021/acs.joc.7b03004. - DOI - PubMed
- Handa N. V.; Li S.; Gerbec J. A.; Sumitani N.; Hawker C. J.; Klinger D. Fully Aromatic High Performance Thermoset via Sydnone–Alkyne Ccloaddition. J. Am. Chem. Soc. 2016, 138, 6400–6403. 10.1021/jacs.6b03381. - DOI - PubMed
-
- Gribble G. W.Mesoionic Ring Systems. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Padwa A., Pearson W. H., Eds.; Wiley & Sons: Hoboken, NJ, 2003; Vol. 59, pp 681–753.
-
- Chang E.-M.; Chen T.-H.; Wong F. F.; Chang E.-C.; Yeh M.-Y. Convenient and efficient synthesis of pyrazole-based DHODase inhibitors from 3-Aryl-4-cyanosydnone. Synlett 2006, 6, 901–904. 10.1055/s-2006-939041. - DOI
- Wu C.; Fang Y.; Larock R. C.; Shi F. Synthesis of 2H-Indazoles by the [3+2] cycloaddition of Arynes and Sydnones. Org. Lett. 2010, 12, 2234–2237. 10.1021/ol100586r. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
