Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer's Disease
- PMID: 31459768
- PMCID: PMC6649263
- DOI: 10.1021/acsomega.9b00198
Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer's Disease
Abstract
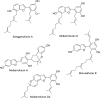
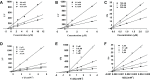
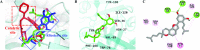
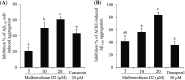
Cholinesterase, β-site amyloid precursor protein cleaving enzyme 1 (BACE1), and glycogen synthase kinase-3β (GSK-3β) are the three main enzymes responsible for the early onset of Alzheimer's disease (AD). The main aim of the present study was to delineate and accentuate the triple-inhibitory potential of arylbenzofurans from Morus alba against these enzymes. Overall, the enzyme inhibition assays demonstrated the prominence of mulberrofuran D2 as an inhibitor of AChE, BChE, BACE1, and GSK-3β enzymes with IC50 values of 4.61, 1.51, 0.73, and 6.36 μM, respectively. Enzyme kinetics revealed different modes of inhibition, and in silico modeling suggested that mulberrofuran D2 inhibited these enzymes with low binding energy through hydrophilic, hydrophobic, and π-cation interactions in the active site cavities. Similarly, in Aβ-aggregation assays, mulberrofuran D2 inhibited self-induced and AChE-induced Aβ aggregation in a concentration-dependent manner that was superior to reference drugs. These results suggest that arylbenzofurans from M. alba, especially mulberrofuran D2, are triple inhibitors of cholinesterase, BACE1, and GSK-3β and may represent a novel class of anti-AD drugs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
