Synthesis of Substituted Isatins from the MBH Adduct of 1,5,6-Trisubstituted Isatins Using (2,4-Dinitrophenyl)hydrazine and K-10 Clay Explored as Protection-Deprotection Chemistry
- PMID: 31460047
- PMCID: PMC6648401
- DOI: 10.1021/acsomega.9b01002
Synthesis of Substituted Isatins from the MBH Adduct of 1,5,6-Trisubstituted Isatins Using (2,4-Dinitrophenyl)hydrazine and K-10 Clay Explored as Protection-Deprotection Chemistry
Abstract
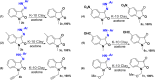
An interesting synthetic transformation of protection-deprotection chemistry in an isatin molecule is achieved. Morita-Baylis-Hillman (MBH) adduct formation used as protection of the C-3 position in the isatin molecule is reported. C-C bond cleavage in the MBH adduct of isatin with the help of phenylhydrazine and C=N bond cleavage in the phenylhydrazone derivative of isatin with the help of K10 clay are studied systematically and reported as deprotection.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



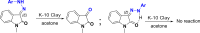



References
-
- Garden S. J.; Skakle J. M. S. Isatin derivatives are reactive electrophilic components for the Baylis–Hillman reaction. Tetrahedron Lett. 2002, 43, 1969–1972. 10.1016/S0040-4039(02)00160-0. - DOI
-
- Chung Y. M.; Im Y. J.; Kim J. N. Baylis-Hillman Reaction of Isatin Derivatives: Isatins as a New Entry for the Baylis-Hillman Reaction. Bull. Korean Chem. Soc. 2002, 23, 1651–1654. 10.5012/bkcs.2002.23.11.1651. - DOI
-
- Kim S. C.; Gowrisankar S.; Kim J. N. Synthesis of 3-aryl-3-hydroxypyrrolidin-2-ones and 2-benzyl-9b-hydroxy-3,3a,5,9b-tetrahydro-2H-pyrrolo[3,4-c]quinoline-1,4-dione derivatives from the Baylis–Hillman adducts of isatins. Tetrahedron Lett. 2006, 47, 3463–3466. 10.1016/j.tetlet.2006.03.074. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

