Mechanism of Thermal Degradation-Induced Gel Formation in Polyamide 6/Ethylene Vinyl Alcohol Blend Nanocomposites Studied by Time-Resolved Rheology and Hyphenated Thermogravimetric Analyzer Fourier Transform Infrared Spectroscopy Mass Spectroscopy: Synergistic Role of Nanoparticles and Maleic-anhydride-Grafted Polypropylene
- PMID: 31460048
- PMCID: PMC6648533
- DOI: 10.1021/acsomega.9b00940
Mechanism of Thermal Degradation-Induced Gel Formation in Polyamide 6/Ethylene Vinyl Alcohol Blend Nanocomposites Studied by Time-Resolved Rheology and Hyphenated Thermogravimetric Analyzer Fourier Transform Infrared Spectroscopy Mass Spectroscopy: Synergistic Role of Nanoparticles and Maleic-anhydride-Grafted Polypropylene
Abstract
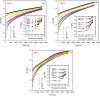
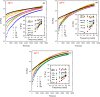
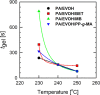
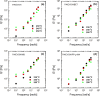
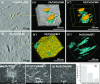
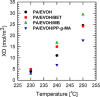
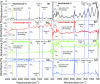
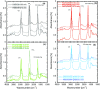
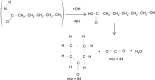
In this study, polyamide 6 (PA) is blended with ethylene vinyl alcohol (EVOH) to yield packaging materials with a balance of mechanical and gas barrier properties. However, the formation of gel-like structures in both polymers because of thermal degradation at high temperatures leads to a processing challenge, particularly during thin-gauge film extrusion. To address this challenge, nanoclays are introduced either directly or via a masterbatch of maleic-anhydride-grafted polypropylene to the PA/EVOH blend and time-resolved rheometry is used to study the effect of different modes of nanoclay incorporation on the kinetics of thermo-oxidative degradation of PA/EVOH blend and its nanocomposites. Time-resolved rheometry measurements allow the acquisition of accurate frequency-dependent linear viscoelastic behavior and offer insights into the rate of degradation or gel formation kinetics and cross-link density. The thermal degradation was studied by thermogravimetric analysis coupled with Fourier transform infrared spectroscopy and mass spectroscopy, allowing the prediction of the possible reactions that take place during the rheological property measurements. The results show that when nanoclays are incorporated directly, the oxidative reactions occur faster. In contrast, in the masterbatch method, oxidative degradation is hindered. The difference in the behaviors is shown to lie in the different nanoclay distributions in the blends; in the blends prepared by the masterbatch method, the nanoclays are dispersed at the interface. In conclusion, the masterbatch-containing blend nanocomposite would benefit processing and product development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







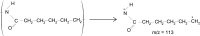


References
-
- Cerruti P.; Laurienzo P.; Malinconico M.; Carfagna C. Thermal oxidative stability and effect of water on gas transport and mechanical properties in PA6-EVOH films. J. Polym. Sci., Part B: Polym. Phys. 2007, 45, 840–849. 10.1002/polb.21095. - DOI
-
- Gorrasi G.; Incarnato L.; Vittoria V.; Acierno D. Structural characterization of nylon 6/EvOH system through transport properties. J. Macromol. Sci., Part B: Phys. 2000, 39, 79–92. 10.1081/MB-100100373. - DOI
-
- Lagaron J. M.; Giménez E.; Saura J. J. Degradation of high barrier ethylene–vinyl alcohol copolymer under mild thermal-oxidative conditions studied by thermal analysis and infrared spectroscopy. Polym. Int. 2001, 50, 635–642. 10.1002/pi.674. - DOI
-
- Yeh J. T.; Yao W. H.; Du Q.; Chen C. C. Blending and barrier properties of blends of modified polyamide and ethylene vinyl alcohol copolymer. J. Polym. Sci., Part B: Polym. Phys. 2005, 43, 511–521. 10.1002/polb.20344. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

