Poly(ethylene glycol)-Based Peptidomimetic "PEGtide" of Oligo-Arginine Allows for Efficient siRNA Transfection and Gene Inhibition
- PMID: 31460100
- PMCID: PMC6647993
- DOI: 10.1021/acsomega.9b00265
Poly(ethylene glycol)-Based Peptidomimetic "PEGtide" of Oligo-Arginine Allows for Efficient siRNA Transfection and Gene Inhibition
Abstract
While a wide range of experimental and commercial transfection reagents are currently available, persistent problems remain regarding their suitability for continued development. These include the transfection efficiency for difficult-to-transfect cell types and the risks of decreased cell viability that may arise from any transfection that does occur. Therefore, research is now turning toward alternative molecules that improve the toxicity profile of the gene delivery vector (GDV), while maintaining the transfection efficiency. Among them, cell-penetrating peptides, such as octa-arginine, have shown significant potential as GDVs. Their pharmacokinetic and pharmacodynamic properties can be enhanced through peptidomimetic conversion, whereby a peptide is modified into a synthetic analogue that mimics its structure and/or function, but whose backbone is not solely based on α-amino acids. Using this technology, novel peptidomimetics were developed by co- and postpolymerization functionalization of substituted ethylene oxides, producing poly(ethylene glycol) (PEG)-based peptidomimetics termed "PEGtides". Specifically, a PEGtide of the poly(α-amino acid) oligo-arginine [poly(glycidylguanidine)] was assessed for its ability to complex and deliver a small interfering ribonucleic acid (siRNA) using a range of cell assays and high-content analysis. PEGtide-siRNA demonstrated significantly increased internalization and gene inhibition over 24 h in Calu-3 pulmonary epithelial cells compared to commercial controls and octa-arginine-treated samples, with no evidence of toxicity. Furthermore, PEGtide-siRNA nanocomplexes can provide significant levels of gene inhibition in "difficult-to-transfect" mouse embryonic hypothalamic (mHypo N41) cells. Overall, the usefulness of this novel PEGtide for gene delivery was clearly demonstrated, establishing it as a promising candidate for continued translational research.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
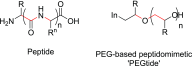



References
-
- Tagalakis A. D.; Munye M. M.; Ivanova R.; Chen H.; Smith C. M.; Aldossary A. M.; Rosa L. Z.; Moulding D.; Barnes J. L.; Kafetzis K. N.; Jones S. A.; Baines D. L.; Moss G. W. J.; O’Callaghan C.; McAnulty R. J.; Hart S. L. Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax 2018, 73, 847–856. 10.1136/thoraxjnl-2017-210670. - DOI - PMC - PubMed
-
- Manunta M. D. I.; Tagalakis A. D.; Attwood M.; Aldossary A. M.; Barnes J. L.; Munye M. M.; Weng A.; McAnulty R. J.; Hart S. L. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: a therapeutic strategy for cystic fibrosis. Sci. Rep. 2017, 7, 70010.1038/s41598-017-00662-2. - DOI - PMC - PubMed
-
- Suhr O. B.; Coelho T.; Buades J.; Pouget J.; Conceicao I.; Berk J.; Schmidt H.; Waddington-Cruz M.; Campistol J. M.; Bettencourt B. R.; Vaishnaw A.; Gollob J.; Adams D. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J. Rare Dis. 2015, 10, 10910.1186/s13023-015-0326-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

