Crystal Structure of FOXC2 in Complex with DNA Target
- PMID: 31460188
- PMCID: PMC6648891
- DOI: 10.1021/acsomega.9b00756
Crystal Structure of FOXC2 in Complex with DNA Target
Abstract
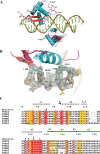
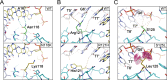
Forkhead transcription factor C2 (FOXC2) is a transcription factor regulating vascular and lymphatic development, and its mutations are linked to lymphedema-distichiasis syndrome. FOXC2 is also a crucial regulator of the epithelial-mesenchymal transition processes essential for tumor metastasis. Here, we report the crystal structure of the FOXC2-DNA-binding domain in complex with its cognate DNA. The crystal structure provides the basis of DNA sequence recognition by FOXC2 for the T/CAAAC motif. Helix 3 makes the majority of the DNA-protein interactions and confers the DNA sequence specificity. The computational energy calculation results also validate the structural observations. The FOXC2 and DNA complex structure provides a detailed picture of protein and DNA interactions, which allows us to predict its DNA recognition specificity and impaired functions in mutants identified in human patients.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


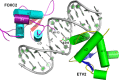
References
-
- Mani S. A.; Guo W.; Liao M.-J.; Eaton E. N.; Ayyanan A.; Zhou A. Y.; Brooks M.; Reinhard F.; Zhang C. C.; Shipitsin M.; Campbell L. L.; Polyak K.; Brisken C.; Yang J.; Weinberg R. A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. 10.1016/j.cell.2008.03.027. - DOI - PMC - PubMed
-
- Mani S. A.; Yang J.; Brooks M.; Schwaninger G.; Zhou A.; Miura N.; Kutok J. L.; Hartwell K.; Richardson A. L.; Weinberg R. A. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 10069–10074. 10.1073/pnas.0703900104. - DOI - PMC - PubMed
-
- Hollier B. G.; Tinnirello A. A.; Werden S. J.; Evans K. W.; Taube J. H.; Sarkar T. R.; Sphyris N.; Shariati M.; Kumar S. V.; Battula V. L.; Herschkowitz J. I.; Guerra R.; Chang J. T.; Miura N.; Rosen J. M.; Mani S. A. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013, 73, 1981–1992. 10.1158/0008-5472.CAN-12-2962. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

