Assembly of Multicomponent Nano-Bioconjugates Composed of Mesoporous Silica Nanoparticles, Proteins, and Gold Nanoparticles
- PMID: 31460202
- PMCID: PMC6647957
- DOI: 10.1021/acsomega.9b01240
Assembly of Multicomponent Nano-Bioconjugates Composed of Mesoporous Silica Nanoparticles, Proteins, and Gold Nanoparticles
Abstract
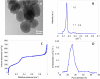
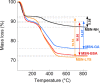
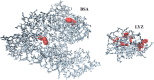
The purpose of this work was the assembly of multicomponent nano-bioconjugates based on mesoporous silica nanoparticles (MSNs), proteins (bovine serum albumin, BSA, or lysozyme, LYZ), and gold nanoparticles (GNPs). These nano-bioconjugates may find applications in nanomedicine as theranostic devices. Indeed, MSNs can act as drug carriers, proteins stabilize MSNs within the bloodstream, or may have therapeutic or targeting functions. Finally, GNPs can either be used as contrast agents for imaging or for photothermal therapy. Here, amino-functionalized MSNs (MSN-NH2) were synthesized and characterized through various techniques (small angle X-rays scattering TEM, N2 adsorption/desorption isotherms, and thermogravimetric analysis (TGA)). BSA or lysozyme were then grafted on the external surface of MSN-NH2 to obtain MSN-BSA and MSN-LYZ bioconjugates, respectively. Protein immobilization on MSNs surface was confirmed by Fourier transform infrared spectroscopy, ζ-potential measurements, and TGA, which also allowed the estimation of protein loading. The MSN-protein samples were then dispersed in a GNP solution to obtain MSN-protein-GNPs nano-bioconjugates. Transmission electron microscopy (TEM) analysis showed the occurrence of GNPs on the MSN-protein surface, whereas almost no GNPs occurred in the protein-free control samples. Fluorescence and Raman spectroscopies suggested that proteins-GNP interactions involve tryptophan residues.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Gold Nanoparticles: A Powerful Tool to Visualize Proteins on Ordered Mesoporous Silica and for the Realization of Theranostic Nanobioconjugates.Int J Mol Sci. 2018 Jul 8;19(7):1991. doi: 10.3390/ijms19071991. Int J Mol Sci. 2018. PMID: 29986530 Free PMC article. Review.
-
Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin.Gels. 2017 Feb 22;3(1):8. doi: 10.3390/gels3010008. Gels. 2017. PMID: 30920505 Free PMC article.
-
Facile Synthesis of Three Types of Mesoporous Silica Microspheres as Drug Delivery Carriers and their Sustained-Release Properties.Curr Drug Deliv. 2023;20(9):1337-1350. doi: 10.2174/1567201819666220616121602. Curr Drug Deliv. 2023. PMID: 35713141
-
Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery.J Biomater Appl. 2017 Oct;32(4):524-532. doi: 10.1177/0885328217724638. Epub 2017 Aug 4. J Biomater Appl. 2017. PMID: 28776488
-
Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents.Molecules. 2020 Aug 21;25(17):3814. doi: 10.3390/molecules25173814. Molecules. 2020. PMID: 32825791 Free PMC article. Review.
Cited by
-
Advances in the application of gold nanoparticles in bone tissue engineering.J Biol Eng. 2020 May 6;14:14. doi: 10.1186/s13036-020-00236-3. eCollection 2020. J Biol Eng. 2020. PMID: 32391080 Free PMC article. Review.
-
Gold Nanoparticles: A Powerful Tool to Visualize Proteins on Ordered Mesoporous Silica and for the Realization of Theranostic Nanobioconjugates.Int J Mol Sci. 2018 Jul 8;19(7):1991. doi: 10.3390/ijms19071991. Int J Mol Sci. 2018. PMID: 29986530 Free PMC article. Review.
-
Gold Nanomaterials for Imaging-Guided Near-Infrared in vivo Cancer Therapy.Front Bioeng Biotechnol. 2019 Dec 5;7:398. doi: 10.3389/fbioe.2019.00398. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31867323 Free PMC article. Review.
-
Recent Advances in Metallic Nanoparticle Assemblies for Surface-Enhanced Spectroscopy.Int J Mol Sci. 2021 Dec 28;23(1):291. doi: 10.3390/ijms23010291. Int J Mol Sci. 2021. PMID: 35008714 Free PMC article. Review.
-
Polythiolactone-Decorated Silica Particles: A Versatile Approach for Surface Functionalization, Catalysis and Encapsulation.Chemistry. 2021 May 17;27(28):7667-7676. doi: 10.1002/chem.202100547. Epub 2021 May 2. Chemistry. 2021. PMID: 33788322 Free PMC article.
References
-
- Chen S.; Zhang Q.; Hou Y.; Zhang J.; Liang X. J. Nanomaterials in Medicine and Pharmaceuticals: Nanoscale Materials Developed with Less Toxicity and More Efficacy. Eur. J. Nanomed. 2013, 5, 61–79. 10.1515/ejnm-2013-0003. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

