First Insight on Small Molecules as Cardiac Calsequestrin Stabilizers
- PMID: 31460256
- PMCID: PMC6682146
- DOI: 10.1021/acsomega.9b01113
First Insight on Small Molecules as Cardiac Calsequestrin Stabilizers
Abstract
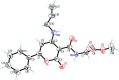
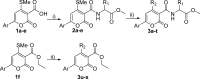
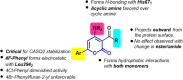
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is caused by mutations of cardiac calsequestrin (CASQ2) that impair its characteristic ability of Ca2+-induced polymerization-depolymerization. However, stabilizing the CASQ2 polymer by pharmacological agents to treat CPVT has not been reported so far. Here, we tested whether small molecules can stabilize CASQ2 polymers. We synthesized 24 glycinate/alaninate/acetate α-pyranone analogs and conducted the CASQ2 depolymerization assay. Most of the molecules of this class of compounds inhibited the depolymerization of the protein upon Ca2+ chelation by ethylene glycol tetraacetic acid. Structure-activity relationship studies revealed that the compounds with the 4-fluoro-phenyl group at the C-6 position of the pyranone ring and open-chain primary amine at C-4 are the most active of the class. This is the first report of an α-pyranone class of compounds with the ability to stabilize CASQ2 polymers and opens up the possibility to target Ca2+-release disorders via modulation of CASQ2 polymerization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


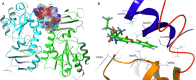
Similar articles
-
The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization.J Biol Chem. 2010 May 28;285(22):17188-96. doi: 10.1074/jbc.M109.096354. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353949 Free PMC article.
-
A novel heterozygous mutation in cardiac calsequestrin causes autosomal dominant catecholaminergic polymorphic ventricular tachycardia.Heart Rhythm. 2016 Aug;13(8):1652-60. doi: 10.1016/j.hrthm.2016.05.004. Epub 2016 May 5. Heart Rhythm. 2016. PMID: 27157848 Free PMC article.
-
Modulation of human ether a gogo related channels by CASQ2 contributes to etiology of catecholaminergic polymorphic ventricular tachycardia (CPVT).Cell Physiol Biochem. 2010;26(4-5):503-12. doi: 10.1159/000322318. Epub 2010 Oct 29. Cell Physiol Biochem. 2010. PMID: 21063088
-
Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights.Cardiovasc Res. 2005 Aug 15;67(3):379-87. doi: 10.1016/j.cardiores.2005.04.027. Cardiovasc Res. 2005. PMID: 15913575 Review.
-
Current topics in catecholaminergic polymorphic ventricular tachycardia.J Arrhythm. 2016 Oct;32(5):344-351. doi: 10.1016/j.joa.2015.09.008. Epub 2015 Nov 24. J Arrhythm. 2016. PMID: 27761157 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

