Reactivity Modes of Cp*M-Type Half-Sandwich Dichalcogenolate Complexes with 2,6-Disubstituted Aryl Azides: The Effects of the Metal Center, Chalcogen, and Ligand Moiety on Product Formation
- PMID: 31460394
- PMCID: PMC6682133
- DOI: 10.1021/acsomega.9b01364
Reactivity Modes of Cp*M-Type Half-Sandwich Dichalcogenolate Complexes with 2,6-Disubstituted Aryl Azides: The Effects of the Metal Center, Chalcogen, and Ligand Moiety on Product Formation
Abstract
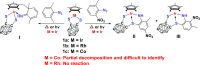
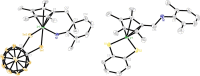
Cp*M-type half-sandwich dichalcogenolate complexes bearing either carborane or benzene moieties show diverse reactivity patterns toward two selected 2,6-disubstituted aryl azides under thermal or photolytic conditions. The chalcogen (S and Se) has little effect on the formation of final products. However, the effects of both the metal center and the ligand moiety of the metal precursor on the reactions were significant. Compared to iridium precursor Cp*IrS2C2B10H10 (1a), rhodium and cobalt analogues (1b: Cp*RhS2C2B10H10, 1c: Cp*CoS2C2B10H10) demonstrated no reactivity toward aryl azides. The reaction of Cp*IrSe2C2B10H10 (1d) with 2,6-Me2C6H3N3 led to the clean formation of complex 2 with C(sp3)-H activation of one methyl group of the Cp* ligand and loss of N2 along with the rearrangement of the benzene ring of the original azide ligand, whereas the treatment of Cp*IrS2C6H4 (1e) with 2,6-Me2C6H3N3 under the same reaction conditions gave a 16-electron half-sandwich complex 5 featuring C-N coupling on one methyl group from the Cp* ligand. When 2-Me-6-NO2C6H3N3 was employed, the same reaction patterns for forming products (3 and 6) with the nitro group migrating to the para-position versus the original aryl azide were observed. In addition, the reaction with metal precursor 1d generated another product 4 featuring the exchange of nitro and azido groups, while the reaction with 1e afforded another complex 7 with the formation of the N-NO2 moiety. All new complexes were characterized by spectroscopy methods, and single-crystal X-ray analyses were performed for complexes 2 and 5-7. Furthermore, radical mechanisms for the formation of complexes 2-7 were proposed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
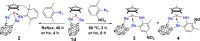
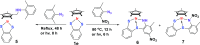

Similar articles
-
Radical coupling for directed C-C/C-S bond formation in the reaction of Cp*IrS2C2B10H10 with 1-azido-3-nitrobenzene.Dalton Trans. 2014 Apr 7;43(13):4962-8. doi: 10.1039/c3dt52308j. Dalton Trans. 2014. PMID: 24108087
-
Reactivity of 16-electron half-sandwich cobalt compounds containing a chelating 1,2-dicarba-closo-dodecaborane-1,2-dithiolate ligand towards methyl propiolate and dithio ligands.Dalton Trans. 2012 Oct 21;41(39):12054-63. doi: 10.1039/c2dt31425h. Dalton Trans. 2012. PMID: 22911065
-
Synthesis and characterization of heterometallic M-Ru (M = Co, Rh, Ir) clusters containing the nido-dicarborane-1,2-dithiolato chelating ligand.Dalton Trans. 2009 Jan 7;(1):111-8. doi: 10.1039/b809520e. Epub 2008 Oct 30. Dalton Trans. 2009. PMID: 19081978
-
Synthesis and reactions of group 6 metal half-sandwich complexes of 2,2-dicyanoethylene-1,1-dichalcogenolates [(Cp*)M[E(2)C=C(CN)(2)](2)]-(M = Mo, W; E = S, Se).Inorg Chem. 2002 Sep 9;41(18):4824-33. doi: 10.1021/ic025609a. Inorg Chem. 2002. PMID: 12206711
-
Transition metal-carboryne complexes: synthesis, bonding, and reactivity.Acc Chem Res. 2011 Apr 19;44(4):299-309. doi: 10.1021/ar100156f. Epub 2011 Mar 11. Acc Chem Res. 2011. PMID: 21395260 Review.
References
-
- Bräse S.; Gil C.; Knepper K.; Zimmermann V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem., Int. Ed. 2005, 44, 5188–5240. 10.1002/anie.200400657. - DOI - PubMed
- Huang D.; Yan G. Recent Advances in Reactions of Azides. Adv. Synth. Catal. 2017, 359, 1600–1619. 10.1002/adsc.201700103. - DOI
-
- Intrieri D.; Zardi P.; Caselli A.; Gallo E. Organic azides: “energetic reagents” for the intermolecular amination of C–H bonds. Chem. Commun. 2014, 50, 11440–11453. 10.1039/c4cc03016h. - DOI - PubMed
- Shin K.; Kim H.; Chang S. Transition-Metal-Catalyzed C-N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C-H Amination. Acc. Chem. Res. 2015, 48, 1040–1052. 10.1021/acs.accounts.5b00020. - DOI - PubMed
- Driver T. G. Recent advances in transition metal-catalyzed N-atom transfer reactions of azides. Org. Biomol. Chem. 2010, 8, 3831–3846. 10.1039/c005219c. - DOI - PMC - PubMed
-
- Cenini S.; La Monica G. Organic azides and isocyanates as sources of nitrene species in organometallic chemistry. Inorg. Chim. Acta 1976, 18, 279–293. 10.1016/s0020-1693(00)95618-4. - DOI
- Cenini S.; Gallo E.; Caselli A.; Ragaini F.; Fantauzzi S.; Piangiolino C. Coordination chemistry of organic azides and amination reactions catalyzed by transition metal complexes. Coord. Chem. Rev. 2006, 250, 1234–1253. 10.1016/j.ccr.2005.10.002. - DOI
-
- Jenkins D. M.; Betley T. A.; Peters J. C. Oxidative Group Transfer to Co(I) Affords a Terminal Co(III) Imido Complex. J. Am. Chem. Soc. 2002, 124, 11238–11239. 10.1021/ja026852b. - DOI - PubMed
- Laskowski C. A.; Miller A. J. M.; Hillhouse G. L.; Cundari T. R. A Two-Coordinate Nickel Imido Complex That Effects C–H Amination. J. Am. Chem. Soc. 2011, 133, 771–773. 10.1021/ja1101213. - DOI - PubMed
- Hu X.; Meyer K. Terminal Cobalt(III) Imido Complexes Supported by Tris(Carbene) Ligands: Imido Insertion into the Cobalt–Carbene Bond. J. Am. Chem. Soc. 2004, 126, 16322–16323. 10.1021/ja044271b. - DOI - PubMed
- Waterman R.; Hillhouse G. L. η2-Organoazide Complexes of Nickel and Their Conversion to Terminal Imido Complexes via Dinitrogen Extrusion. J. Am. Chem. Soc. 2008, 130, 12628–12629. 10.1021/ja805530z. - DOI - PubMed
- Harrold N. D.; Waterman R.; Hillhouse G. L.; Cundari T. R. Group-Transfer Reactions of Nickel–Carbene and −Nitrene Complexes with Organoazides and Nitrous Oxide that Form New C=N, C=O, and N=N Bonds. J. Am. Chem. Soc. 2009, 131, 12872–12873. 10.1021/ja904370h. - DOI - PubMed
- Iluc V. M.; Miller A. J. M.; Anderson J. S.; Monreal M. J.; Mehn M. P.; Hillhouse G. L. Synthesis and Characterization of Three-Coordinate Ni(III)-Imide Complexes. J. Am. Chem. Soc. 2011, 133, 13055–13063. 10.1021/ja2024993. - DOI - PMC - PubMed
- Wu H.; Hall M. B. A New Mechanism for the Conversion of Transition Metal Azides to Imido Complexes. J. Am. Chem. Soc. 2008, 130, 16452–16453. 10.1021/ja805105q. - DOI - PubMed
- Travia N. E.; Xu Z.; Keith J. M.; Ison E. A.; Fanwick P. E.; Hall M. B.; Abu-Omar M. M. Observation of Inductive Effects That Cause a Change in the Rate-Determining Step for the Conversion of Rhenium Azides to Imido Complexes. Inorg. Chem. 2011, 50, 10505–10514. 10.1021/ic2017853. - DOI - PubMed
- Geer A. M.; Tejel C.; López J. A.; Ciriano M. A. Terminal Imido Rhodium Complexes. Angew. Chem., Int. Ed. 2014, 53, 5614–5618. 10.1002/anie.201400023. - DOI - PubMed
- Bellow J. A.; Yousif M.; Cabelof A. C.; Lord R. L.; Groysman S. Reactivity Modes of an Iron Bis(alkoxide) Complex with Aryl Azides: Catalytic Nitrene Coupling vs Formation of Iron(III) Imido Dimers. Organometallics 2015, 34, 2917–2923. 10.1021/acs.organomet.5b00231. - DOI
- Liu Y.; Du J.; Deng L. Synthesis, Structure, and Reactivity of Low-Spin Cobalt(II) Imido Complexes [(Me3P)3Co(NAr)]. Inorg. Chem. 2017, 56, 8278–8286. 10.1021/acs.inorgchem.7b00941. - DOI - PubMed
-
- Brown S. D.; Betley T. A.; Peters J. C. A Low-Spin d5 Iron Imide: Nitrene Capture by Low-Coordinate Iron(I) Provides the 4-Coordinate Fe(III) Complex [PhB(CH2PPh2)3]Fe⋮N-p-tolyl. J. Am. Chem. Soc. 2003, 125, 322–323. 10.1021/ja028448i. - DOI - PubMed
- Guillemot G.; Solari E.; Floriani C.; Rizzoli C. Nitrogen-to-Metal Multiple Bond Functionalities: The Reaction of Calix[4]arene–W(IV) with Azides and Diazoalkanes. Organometallics 2001, 20, 607–615. 10.1021/om000612s. - DOI
- Manßen M.; Weimer I.; Adler C.; Fischer M.; Schmidtmann M.; Beckhaus R. From Organic Azides through Titanium Triazenido Complexes to Titanium Imides. Eur. J. Inorg. Chem. 2018, 131–136. 10.1002/ejic.201701273. - DOI
- Harman W. H.; Lichterman M. F.; Piro N. A.; Chang C. J. Well-Defined Vanadium Organoazide Complexes and Their Conversion to Terminal Vanadium Imides: Structural Snapshots and Evidence for a Nitrene Capture Mechanism. Inorg. Chem. 2012, 51, 10037–10042. 10.1021/ic301673g. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

