NMR Assays for Estimating the O-Acetyl Content of Meningococcal Polysaccharide Serogroup A in Quadrivalent Conjugate Vaccine Formulation
- PMID: 31460407
- PMCID: PMC6681974
- DOI: 10.1021/acsomega.9b01678
NMR Assays for Estimating the O-Acetyl Content of Meningococcal Polysaccharide Serogroup A in Quadrivalent Conjugate Vaccine Formulation
Erratum in
-
Correction to "NMR Assays for Estimating the O-Acetyl Content of Meningococcal Polysaccharide Serogroup A in Quadrivalent Conjugate Vaccine Formulation".ACS Omega. 2019 Sep 10;4(13):15771. doi: 10.1021/acsomega.9b02774. eCollection 2019 Sep 24. ACS Omega. 2019. PMID: 31572881 Free PMC article.
Abstract
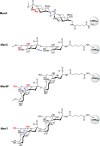
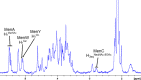
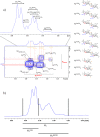
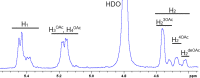
The use of multivalent glycoconjugate vaccines has dramatically contributed to reduce the incidence of meningococcal infectious disease. The advanced structural characterization of polysaccharide conjugates leads to enhancements in the quality and control of the products. Here, we report a novel nuclear magnetic resonance (NMR) method to confirm the identity and structural conformity (e.g., O-acetyl content) of saccharide antigens that comprise a licensed tetravalent meningococcal serogroups A, C, W, and Y vaccine. For the first time, the NMR methodology is applied on a formulation (licensed vaccine) containing a large excess of excipient (i.e., sucrose) without analytical sample pretreatment. This work confirms the applicability of a rapid and easy NMR assay on a multivalent conjugate vaccine, which might be extended to other combination vaccines that are already licensed or in clinical development.
Conflict of interest statement
The authors declare the following competing financial interest(s): All the authors are employees of the GSK companies. Francesco Berti (corresponding author) is owner of patents on related topics. This study was sponsored by GSK Biologicals SA.
Figures
Similar articles
-
Glycoconjugate content quantification to assess vaccine potency: A simplified approach.Biologicals. 2022 Apr;76:10-14. doi: 10.1016/j.biologicals.2022.02.004. Epub 2022 Mar 6. Biologicals. 2022. PMID: 35264299
-
A randomized trial to assess safety and immunogenicity of alternative formulations of a quadrivalent meningococcal (A, C, Y, and W-135) tetanus protein conjugate vaccine in toddlers.Pediatr Infect Dis J. 2012 Jan;31(1):e15-23. doi: 10.1097/INF.0b013e31823e1e34. Pediatr Infect Dis J. 2012. PMID: 22094636 Clinical Trial.
-
Quantitation of serogroups in multivalent polysaccharide-based meningococcal vaccines: optimisation of hydrolysis conditions and chromatographic methods.Vaccine. 2013 Aug 12;31(36):3702-11. doi: 10.1016/j.vaccine.2013.05.098. Epub 2013 Jun 10. Vaccine. 2013. PMID: 23764533
-
Analytical Challenges in Novel Pentavalent Meningococcal Conjugate Vaccine (A, C, Y, W, X).Vaccines (Basel). 2024 Oct 29;12(11):1227. doi: 10.3390/vaccines12111227. Vaccines (Basel). 2024. PMID: 39591130 Free PMC article. Review.
-
Meningococcal glycoconjugate vaccines.Hum Vaccin. 2011 Feb;7(2):170-82. doi: 10.4161/hv.7.2.13717. Epub 2011 Feb 1. Hum Vaccin. 2011. PMID: 21178398 Free PMC article. Review.
Cited by
-
Evolution of Vaccines Formulation to Tackle the Challenge of Anti-Microbial Resistant Pathogens.Int J Mol Sci. 2023 Jul 27;24(15):12054. doi: 10.3390/ijms241512054. Int J Mol Sci. 2023. PMID: 37569427 Free PMC article. Review.
-
Development and Immunogenicity of a Brazilian Glycoconjugate vaccine against Meningococcal W in a Pilot Scale.Glycoconj J. 2021 Oct;38(5):539-549. doi: 10.1007/s10719-021-10016-w. Epub 2021 Sep 13. Glycoconj J. 2021. PMID: 34515909
References
-
- Berti F.Multivalent meningococcal conjugate vaccines: chemical conjugation strategies used for the preparation of vaccines licensed or in clinical trials. In Carbohydrate-Based Vaccines: From Concept to Clinic, ACS Symposium Series, 2018; Vol. 1290, chapter 6, pp 123–137.
-
- Chen W. H.; Neuzil K. M.; Boyce C. R.; Pasetti M. F.; Reymann M. K.; Martellet L.; Hosken N.; LaForce F. M.; Dhere R. M.; Pisal S. S.; Chaudhari A.; Kulkarni P. S.; Borrow R.; Findlow H.; Brown V.; McDonough M. L.; Dally L.; Alderson M. R. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect. Dis. 2018, 18, 1088–1096. 10.1016/s1473-3099(18)30400-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
