Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species
- PMID: 31460742
- PMCID: PMC7007013
- DOI: 10.1021/acs.accounts.9b00302
Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species
Abstract
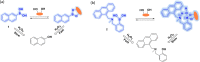
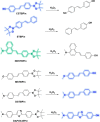
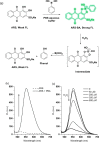
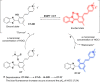
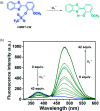
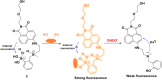
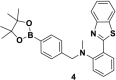
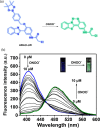
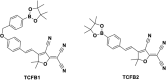
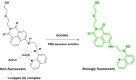
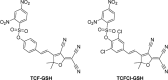
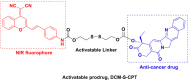
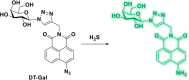
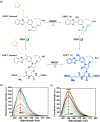
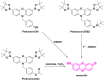
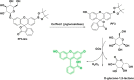
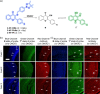
This Account describes a range of strategies for the development of fluorescent probes for detecting reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive (redox-active) sulfur species (RSS). Many ROS/RNS have been implicated in pathological processes such as Alzheimer's disease, cancer, diabetes mellitus, cardiovascular disease, and aging, while many RSS play important roles in maintaining redox homeostasis, serving as antioxidants and acting as free radical scavengers. Fluorescence-based systems have emerged as one of the best ways to monitor the concentrations and locations of these often very short lived species. Because of the high levels of sensitivity and in particular their ability to be used for temporal and spatial sampling for in vivo imaging applications. As a direct result, there has been a huge surge in the development of fluorescent probes for sensitive and selective detection of ROS, RNS, and RSS within cellular environments. However, cellular environments are extremely complex, often with more than one species involved in a given biochemical process. As a result, there has been a rise in the development of dual-responsive fluorescent probes (AND-logic probes) that can monitor the presence of more than one species in a biological environment. Our aim with this Account is to introduce the fluorescent probes that we have developed for in vitro and in vivo measurement of ROS, RNS, and RSS. Fluorescence-based sensing mechanisms used in the construction of the probes include photoinduced electron transfer, intramolecular charge transfer, excited-state intramolecular proton transfer (ESIPT), and fluorescence resonance energy transfer. In particular, probes for hydrogen peroxide, hypochlorous acid, superoxide, peroxynitrite, glutathione, cysteine, homocysteine, and hydrogen sulfide are discussed. In addition, we describe the development of AND-logic-based systems capable of detecting two species, such as peroxynitrite and glutathione. One of the most interesting advances contained in this Account is our extension of indicator displacement assays (IDAs) to reaction-based indicator displacement assays (RIAs). In an IDA system, an indicator is allowed to bind reversibly to a receptor. Then a competitive analyte is introduced into the system, resulting in displacement of the indicator from the host, which in turn modulates the optical signal. With an RIA-based system, the indicator is cleaved from a preformed receptor-indicator complex rather than being displaced by the analyte. Nevertheless, without a doubt the most significant result contained in this Account is the use of an ESIPT-based probe for the simultaneous sensing of fibrous proteins/peptides AND environmental ROS/RNS.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
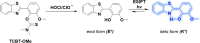


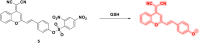

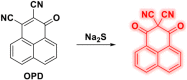

References
-
- Winyard P. G.; Ryan B.; Eggleton P.; Nissim A.; Taylor E.; Lo Faro M. L.; Burkholz T.; Szabó-Taylor K. E.; Fox B.; Viner N.; Haigh R. C.; Benjamin N.; Jones A. M.; Whiteman M. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem. Soc. Trans. 2011, 39 (5), 1226–1232. 10.1042/BST0391226. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

