Modular Synthesis of Di- and Trisubstituted Imidazoles from Ketones and Aldehydes: A Route to Kinase Inhibitors
- PMID: 31460764
- PMCID: PMC6829625
- DOI: 10.1021/acs.joc.9b01844
Modular Synthesis of Di- and Trisubstituted Imidazoles from Ketones and Aldehydes: A Route to Kinase Inhibitors
Abstract
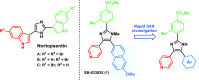
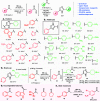
A one-pot and modular approach to the synthesis of 2,4(5)-disubstituted imidazoles was developed based on ketone oxidation, employing catalytic HBr and DMSO, followed by imidazole condensation with aldehydes. This methodology afforded twenty-nine disubstituted NH-imidazoles (23%-85% yield). A three-step synthesis of 20 kinase inhibitors was achieved by employing this oxidation-condensation protocol, followed by bromination and Suzuki coupling in the imidazole ring to yield trisubstituted NH-imidazoles (23%-69%, three steps). This approach was also employed in the synthesis of known inhibitor GSK3037619A.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Anderson E. B.; Long T. E. Imidazole- and Imidazolium-Containing Polymers for Biology and Material Science Applications. Polymer 2010, 51 (12), 2447–2454. 10.1016/j.polymer.2010.02.006. - DOI
-
- Green M. D.; Long T. E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49 (4), 291–314. 10.1080/15583720903288914. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

