A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial
- PMID: 31461239
- PMCID: PMC6868495
- DOI: 10.1093/eurheartj/ehz427
A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial
Abstract
Aims: Stroke is often a devastating event among patients with heart failure with reduced ejection (HFrEF). In COMMANDER HF, rivaroxaban 2.5 mg b.i.d. did not reduce the composite of first occurrence of death, stroke, or myocardial infarction compared with placebo in patients with HFrEF, coronary artery disease (CAD), and sinus rhythm. We now examine the incidence, timing, type, severity, and predictors of stroke or a transient ischaemic attack (TIA), and seek to establish the net clinical benefit of treatment with low-dose rivaroxaban.
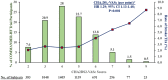
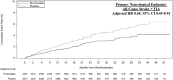
Methods and results: In this double-blind, randomized trial, 5022 patients who had HFrEF(≤40%), elevated natriuretic peptides, CAD, and who were in sinus rhythm were treated with rivaroxaban 2.5 mg b.i.d. or placebo in addition to antiplatelet therapy, after an episode of worsening HF. The primary neurological outcome for this post hoc analysis was time to first event of any stroke or TIA. Over a median follow-up of 20.5 (25th-75th percentiles 20.0-20.9) months, 150 all-cause stroke (127) or TIA (23) events occurred (ischaemic stroke in 82% and haemorrhagic stroke in 11% of stroke events). Overall, 47.5% of first-time strokes were either disabling (16.5%) or fatal (31%). Prior stroke, low body mass index, geographic region, and the CHA2DS2-VASc score were predictors of stroke/TIA. Rivaroxaban significantly reduced the primary neurological endpoint of all-cause stroke or TIA compared with placebo by 32% (1.29 events vs. 1.90 events per 100 patient-years), adjusted for the time from index HF event to randomization and stratified by geographic region (adjusted hazard ratio 0.68, 95% confidence interval 0.49-0.94), with a number needed to treat of 164 patients per year to prevent one stroke/TIA event. The principal safety endpoint of fatal bleeding or bleeding into a critical space, occurred at a similar rate on rivaroxaban and placebo (0.44 events vs. 0.55 events per 100 patient-years).
Conclusions: Patients with HFrEF and CAD are at risk for stroke or TIA in the period following an episode of worsening heart failure in the absence of atrial fibrillation. Most strokes are of ischaemic origin and nearly half are either disabling or fatal. Rivaroxaban at a dose of 2.5 mg b.i.d. reduced rates of stroke or TIA compared with placebo in this population.
Trial registration: COMMANDER HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure); ClinicalTrials.gov NCT01877915.
Keywords: Heart failure; Oral anticoagulation; Stroke; Thrombotic; Transient ischaemic attack.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


Comment in
-
Stroke prevention by low-dose anticoagulation in patients with heart failure and sinus rhythm: is it worth the effort?Eur Heart J. 2019 Nov 21;40(44):3602-3604. doi: 10.1093/eurheartj/ehz523. Eur Heart J. 2019. PMID: 31369067 No abstract available.
References
-
- Vaduganathan M, Patel RB, Yancy CW.. Stroke prevention in heart failure and sinus rhythm: where do we go from here? Eur J Heart Fail 2016;18:1267–1269. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS.. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. - PubMed
-
- Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY.. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015;314:1030–1038. - PubMed
-
- Ferreira JP, Girerd N, Gregson J, Latar I, Sharma A, Pfeffer MA, McMurray JJV, Abdul-Rahim AH, Pitt B, Dickstein K, Rossignol P, Zannad F.. Stroke risk in patients with reduced ejection fraction after myocardial infarction without atrial fibrillation. J Am Coll Cardiol 2018;71:727–735. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

