Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis
- PMID: 31461386
- PMCID: PMC6902677
- DOI: 10.1096/fj.201802805RR
Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis
Abstract
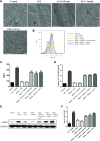
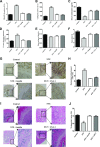
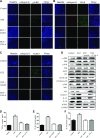
Diabetes mellitus (DM) affects bone metabolism and leads to osteoporosis; however, its pathogenetic mechanisms remain unknown. We found that high glucose (HG) conditions induced the production of reactive oxygen species (ROS) and the expression of proteins related to MAPKs [phosphorylated (p)-ERK, p-JNK, and p-p38], NF-κB (NF-κB, p-IκB, and IKK), and NACHT-LRR-PYD domains-containing protein 3 (NALP3) (NLRP3) [apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), caspase-1, IL-18, IL-1β, and NLRP3] in osteoclasts (OCs) in vitro. Further analysis showed that in HG-induced OCs, ROS is an upstream signal for MAPKs, NF-κB, and the NLRP3 inflammasome. Moreover, MAPKs mediated the activation of NF-κB and NLRP3, whereas NF-κB up-regulated the NLRP3 inflammasome response. Interestingly, HG inducement enhanced the bone resorption of OCs but inhibited their efferocytosis, whereas insulin and lipoxin A4 (4) treatment reversed this phenomenon. In streptozotocin-induced diabetic rats in vivo, the numbers and the bone-resorption capacity of OCs as well as the serum levels of TRACP-5b were significantly increased, and the expression of MAPK-, NF-κB-, and NLRP3 inflammasome-related proteins in the proximal tibia were also significantly elevated; however, treatment with insulin and LXA4 reversed this elevation. Together, these results demonstrated that the activation of ROS/MAPKs/NF-κB/NLRP3 and the inhibition of efferocytosis in OCs are the main causes of osteoporosis in DM.-An, Y., Zhang, H., Wang, C., Jiao, F., Xu, H., Wang, X., Luan, W., Ma, F., Ni, L., Tang, X., Liu, M., Guo, W., Yu, L. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis.
Keywords: diabetes mellitus osteoporosis; infalmmasome; osteoclasts; reactive oxygen species.
Conflict of interest statement
The authors thank Dr. Yang Wang (Jilin University) for her help in manuscript revision. This work was supported by the Project of Science and Technology Department of Jilin Province (20180414036GH); the Education Department of Jilin Province (2016444); the State Key Project of Research and Development Plan (2017YFD0502200 and 2016YFD0501302); the National Nature Science Foundation of China (31172364 and 81801972); the Fund for Science and Technology Development of Jilin Province (20150101108JC); the Important National Science and Technology Specific Projects (2012ZX10003002); the Program for New Century Excellent Talents in University (NCET-09-0434); and the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ2016031100720906 and JSGG20160301100442775). The authors declare no conflicts of interest.
Figures


References
-
- Bain S., Ramamurthy N. S., Impeduglia T., Scolman S., Golub L. M., Rubin C. (1997) Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone 21, 147–153 - PubMed
-
- Hie M., Yamazaki M., Tsukamoto I. (2009) Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 621, 1–9 - PubMed
-
- Saito M., Marumo K. (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 21, 195–214 - PubMed
-
- Lu H., Kraut D., Gerstenfeld L. C., Graves D. T. (2003) Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144, 346–352 - PubMed
-
- Vestergaard P. (2009) Bone metabolism in type 2 diabetes and role of thiazolidinediones. Curr. Opin. Endocrinol. Diabetes Obes. 16, 125–131 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

