Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model
- PMID: 31461505
- PMCID: PMC6713395
- DOI: 10.1371/journal.pone.0221828
Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model
Abstract
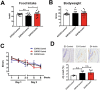
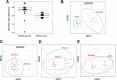
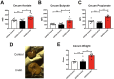
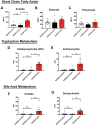
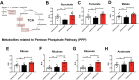
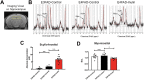
The apolipoprotein ε4 allele (APOE4) is the strongest genetic risk factor for Alzheimer's disease (AD). APOE4 carriers develop systemic metabolic dysfunction decades before showing AD symptoms. Accumulating evidence shows that the metabolic dysfunction accelerates AD development, including exacerbated amyloid-beta (Aβ) retention, neuroinflammation and cognitive decline. Therefore, preserving metabolic function early on may be critical to reducing the risk for AD. Here, we show that inulin increases beneficial microbiota and decreases harmful microbiota in the feces of young, asymptomatic APOE4 transgenic (E4FAD) mice and enhances metabolism in the cecum, periphery and brain, as demonstrated by increases in the levels of SCFAs, tryptophan-derived metabolites, bile acids, glycolytic metabolites and scyllo-inositol. We show that inulin also reduces inflammatory gene expression in the hippocampus. This knowledge can be utilized to design early precision nutrition intervention strategies that use a prebiotic diet to enhance systemic metabolism and may be useful for reducing AD risk in asymptomatic APOE4 carriers.
Conflict of interest statement
Scott McCulloch is employed by Metabolon Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

