1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis
- PMID: 31461622
- PMCID: PMC7610657
- DOI: 10.1021/jacs.9b07564
1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis
Abstract
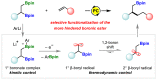
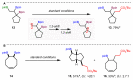
1,2-Bis-boronic esters are versatile intermediates that enable the rapid elaboration of simple alkene precursors. Previous reports on their selective mono-functionalization have targeted the most accessible position, retaining the more hindered secondary boronic ester. In contrast, we have found that photoredox-catalyzed mono-deboronation generates primary β-boryl radicals that undergo rapid 1,2-boron shift to form thermodynamically favored secondary radicals, allowing for selective transformation of the more hindered boronic ester. The pivotal 1,2-boron shift, which has been demonstrated to be stereoretentive, enables access to a wide range of functionalized boronic esters and has been applied to highly diastereoselective fragmentation and transannular cyclization reactions. Furthermore, its generality has been shown in a radical cascade reaction with an allylboronic ester.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Shaw MH, Twilton J, MacMillan DWC. Photoredox Catalysis in Organic Chemistry. J Org Chem. 2016;81:6898. - PMC - PubMed
- Narayanama JMR, Stephenson CRJ. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem Soc Rev. 2011;40:102. - PubMed
- Prier CK, Rankic DA, MacMillan DWC. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem Rev. 2013;113:5322. - PMC - PubMed
- Goddard J-P, Ollivier C, Fensterbank L. Photoredox Catalysis for the Generation of Carbon Centered Radicals. Acc Chem Res. 2016;49:1924. - PubMed
- Staveness D, Bosque I, Stephenson CRJ. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc Chem Res. 2016;49:2295. - PMC - PubMed
- Marzo L, Pagire SK, Reiser O, König B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew Chem Int Ed. 2018;57:10034. - PubMed
-
- Yasu Y, Koike T, Akita M. Visible Light-Induced Selective Generation of Radicals from Organoborates by Photoredox Catalysis. Adv Synth Catal. 2012;354:3414.
- Lima F, Sharma UK, Grunenberg L, Saha D, Johannsen S, Sedelmeier J, Van der Eycken EV, Ley SV. A Lewis Base Catalysis Approach for the Photoredox Activation of Boronic Acids and Esters. Angew Chem Int Ed. 2017;56:15136. - PMC - PubMed
- Lima F, Grunenberg L, Rahman HBA, Labes R, Sedelmeier J, Ley SV. Organic Photocatalysis for the Radical Couplings of Boronic Acid Derivatives in Batch and Flow. Chem Commun. 2018;54:5606. - PubMed
- Shu C, Noble A, Aggarwal VK. Photoredox-Catalyzed Cyclobutane Synthesis by a Deboronative Radical Addition-Polar Cyclization Cascade. Angew Chem Int Ed. 2019;58:3870. - PMC - PubMed
- Lima F, Kabeshov MA, Tran DN, Battilocchio C, Sedelmeier J, Sedelmeier G, Schenkel B, Ley SV. Visible Light Activation of Boronic Esters Enables Efficient Photoredox C(sp2)-C(sp3) Cross-Couplings in Flow. Angew Chem Int Ed. 2016;55:14085. - PMC - PubMed
- Clausen F, Kischkewitz M, Bergander K, Studer A. Catalytic Protodeboronation of Pinacol Boronic Esters: Formal Anti-Markovnikov Hydromethylation of Alkenes. Chem Sci. 2019;10:6210. - PMC - PubMed
- Duret G, Quinlan R, Bisseret P, Blanchard N. Boron Chemistry in a New Light. Chem Sci. 2015;6:5366. - PMC - PubMed
-
- Miyazawa K, Yasu Y, Koike T, Akita M. Visible-Light-Induced Hydroalkoxymethylation of Electron-Deficient Alkenes by Photoredox Catalysis. Chem Commun. 2013;49:7249. - PubMed
- Miyazawa K, Koike T, Akita M. Hydroaminomethylation of Olefins with Aminomethyltrifluoroborate by Photoredox Catalysis. Adv Synth Catal. 2014;356:2749.
- Chinzei T, Miyazawa K, Yasu Y, Koike T, Akita M. Redox-Economical Radical Generation from Organoborates and Carboxylic Acids by Organic Photoredox Catalysis. RSC Adv. 2015;5:21297.
- Huo H, Harms K, Meggers E. Catalytic, Enantioselective Addition of Alkyl Radicals to Alkenes via Visible-Light-Activated Photoredox Catalysis with a Chiral Rhodium Complex. J Am Chem Soc. 2016;138:6936. - PubMed
- Tellis JC, Primer DN, Molander GA. Single-Electron Transmetalation in Or-ganoboron Cross-Coupling by Photoredox/Nickel Dual Catalysis. Science. 2014;345:433. - PMC - PubMed
- Primer DN, Karakaya I, Tellis JC, Molander GA. Single-Electron Transmetalation: An Enabling Technology for Secondary Alkylboron Cross-Coupling. J Am Chem Soc. 2015;137:2195. - PMC - PubMed
- Primer DN, Molander GA. Enabling the Cross-Coupling of Tertiary Organoboron Nucleophiles through Radical-Mediated Alkyl Transfer. J Am Chem Soc. 2017;139:9847. - PMC - PubMed
- Amani J, Molander GA. Synergistic Photoredox/Nickel Coupling of Acyl Chlorides with Secondary Alkyltrifluoroborates: Dialkyl Ketone Synthesis. J Org Chem. 2017;82:1856. - PMC - PubMed
- Huang H, Zhang G, Gong L, Zhang S, Chen Y. Visible-Light-Induced Chemoselective Deboronative Alkynylation under Biomolecule-Compatible Conditions. J Am Chem Soc. 2014;136:2280. - PubMed
- Huang H, Jia K, Chen Y. Hypervalent Iodine Reagents Enable Chemoselective Deboronative/Decarboxylative Alkenylation by Photoredox Catalysis. Angew Chem Int Ed. 2015;54:1881. - PubMed
- Heitz DR, Rizwan K, Molander GA. Visible-Light-Mediated Alkenylation, Allylation, and Cyanation of Potassium Alkyltrifluoroborates with Organic Photoredox Catalysts. J Org Chem. 2016;81:7308. - PMC - PubMed
- Lang SB, Wiles RJ, Kelly CB, Molander GA. Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics. Angew Chem Int Ed. 2017;56:15073. - PMC - PubMed
-
- Bonet A, Pubill-Ulldemolins C, Bo C, Gulyás H, Fernández E. Transition-Metal-Free Diboration Reaction by Activation of Diboron Compounds with Simple Lewis Bases. Angew Chem Int Ed. 2011;50:7158. - PubMed
- Miralles N, Cid J, Cuenca AB, Carbó JJ, Fernández E. Mixed Diboration of Alkenes in a Metal-Free Context. Chem Commun. 2015;51:1693. - PubMed
- Farre A, Soares K, Briggs RA, Balanta A, Benoit DM, Bonet A. Amine Catalysis for the Organocatalytic Diboration of Challenging Alkenes. Chem Eur J. 2016;22:17552. - PubMed
- Farre A, Briggs R, Pubill-Ulldemolins C, Bonet A. Developing a Bench-Scale Green Diboration Reaction toward Industrial Application. Synthesis. 2017;49:4775.
- Bonet A, Sole C, Gulyás H, Fernández E. Asymmetric Organocatalytic Diboration of Alkenes. Org Biomol Chem. 2012;10:6621. - PubMed
- Blaisdell TP, Caya TC, Zhang L, Sanz-Marco A, Morken JP. Hydroxyl-Directed Stereoselective Diboration of Alkenes. J Am Chem Soc. 2014;136:9264. - PMC - PubMed
- Fang L, Yan L, Haeffner F, Morken JP. Carbohydrate-Catalyzed Enantioselective Alkene Diboration: Enhanced Reactivity of 1,2-Bonded Diboron Complexes. J Am Chem Soc. 2016;138:2508. - PMC - PubMed
- Yan L, Meng Y, Haeffner F, Leon RM, Crockett MP, Morken JP. Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope. J Am Chem Soc. 2018;140:3663. - PMC - PubMed
- Morgan JB, Miller SP, Morken JP. Rhodium-Catalyzed Enantioselective Diboration of Simple Alkenes. J Am Chem Soc. 2003;125:8702. - PubMed
- Trudeau S, Morgan JB, Shrestha M, Morken JP. Rh-Catalyzed Enantioselective Diboration of Simple Alkenes: Reaction Development and Substrate Scope. J Org Chem. 2005;70:9538. - PubMed
- Kliman LT, Mlynarski SN, Morken JP. Pt-Catalyzed Enantioselective Diboration of Terminal Alkenes with B2(pin)2. J Am Chem Soc. 2009;131:13210. - PMC - PubMed
- Coombs JR, Haeffner F, Kliman LT, Morken JP. Scope and Mechanism of the Pt-Catalyzed Enantioselective Diboration of Monosubstituted Alkenes. J Am Chem Soc. 2013;135:11222. - PMC - PubMed
- Toribatake K, Nishiyama H. Asymmetric Diboration of Terminal Alkenes with a Rhodium Catalyst and Subsequent Oxidation: Enantioselective Synthesis of Optically Active 1,2-Diols. Angew Chem Int Ed. 2013;52:11011. - PubMed
- Toribatake K, Nishiyama H, Miyata S, Naganawa Y, Nishiyama H. Asymmetric Synthesis of Optically Active 3-Amino-1,2-Diols from N-Acyl-Protected Allylamines via Catalytic Diboration with Rh[bis(oxazolinyl)phenyl] Catalysts. Tetrahedron. 2015;71:3203.
-
- Sandford C, Aggarwal VK. Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem Commun. 2017;53:5481. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

