VE-Cadherin Is Required for Lymphatic Valve Formation and Maintenance
- PMID: 31461654
- PMCID: PMC6743082
- DOI: 10.1016/j.celrep.2019.07.072
VE-Cadherin Is Required for Lymphatic Valve Formation and Maintenance
Abstract
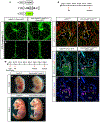
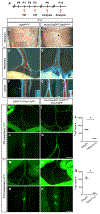
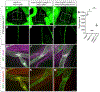
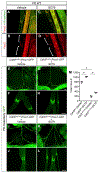
The lymphatic vasculature requires intraluminal valves to maintain forward lymph flow. Lymphatic valves form and are constantly maintained by oscillatory fluid flow throughout life, yet the earliest steps of how lymphatic endothelial cells are able to respond to fluid shear stress remain unknown. Here, we show that the adherens junction protein VE-cadherin is required for the upregulation of valve-specific transcription factors. Conditional deletion of VE-cadherin in vivo prevented valve formation in the embryo and caused postnatal regression of nearly all lymphatic valves in multiple tissues. Since VE-cadherin is known to signal through β-catenin and the VEGFR/AKT pathway, each pathway was probed. Expression of a constitutively active β-catenin mutant or direct pharmacologic activation of AKT in vivo significantly rescued valve regression in the VE-cadherin-deficient lymphatic vessels. In conclusion, VE-cadherin-dependent signaling is required for lymphatic valve formation and maintenance and therapies to augment downstream pathways hold potential to treat lymphedema in patients.
Keywords: Akt; mechanotransduction; shear stress; β-catenin.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

