Quality Analysis of Minerals Formed by Jaw Periosteal Cells under Different Culture Conditions
- PMID: 31461878
- PMCID: PMC6747376
- DOI: 10.3390/ijms20174193
Quality Analysis of Minerals Formed by Jaw Periosteal Cells under Different Culture Conditions
Abstract
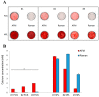
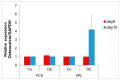
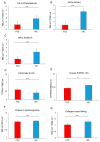
Previously, we detected a higher degree of mineralization in fetal calf serum (FCS) compared to serum-free cultured jaw periosteum derived osteoprogenitor cells (JPCs). By Raman spectroscopy, we detected an earlier formation of mineralized extracellular matrix (ECM) of higher quality under serum-free media conditions. However, mineralization potential remained too low. In the present study, we aimed to investigate the biochemical composition and subsequent biomechanical properties of the JPC-formed ECM and minerals under human platelet lysate (hPL) and FCS supplementation. JPCs were isolated (n = 4 donors) and expanded under FCS conditions and used in passage five for osteogenic induction under both, FCS and hPL media supplementation. Raman spectroscopy and Alizarin Red/von Kossa staining were employed for biochemical composition analyses and for visualization and quantification of mineralization. Osteocalcin gene expression was analyzed by quantitative PCR. Biomechanical properties were assessed by using atomic force microscopy (AFM). Raman spectroscopic measurements showed significantly higher (p < 0.001) phosphate to protein ratios and in the tendency, lower carbonate to phosphate ratios in osteogenically induced JPCs under hPL in comparison to FCS culturing. Furthermore, higher crystal sizes were detected under hPL culturing of the cells. With respect to the ECM, significantly higher ratios of the precursor protein proline to hydroxyproline were detected in hPL-cultured JPC monolayers (p < 0.001). Additionally, significantly higher levels (p < 0.001) of collagen cross-linking were calculated, indicating a higher degree of collagen maturation in hPL-cultured JPCs. By atomic force microscopy, a significant increase in ECM stiffness (p < 0.001) of FCS cultured JPC monolayers was observed. The reverse effect was measured for the JPC formed precipitates/minerals. Under hPL supplementation, JPCs formed minerals of significantly higher stiffness (p < 0.001) when compared to the FCS setting. This study demonstrates that hPL culturing of JPCs leads to the formation of an anorganic material of superior quality in terms of biochemical composition and mechanical properties.
Keywords: Raman spectroscopy; atomic force microscopy; bone mineral formation; fetal calf serum; human platelet lysate; mechanical properties; osteoprogenitor jaw periosteal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Jaw Periosteum-Derived Mesenchymal Stem Cells Regulate THP-1-Derived Macrophage Polarization.Int J Mol Sci. 2021 Apr 21;22(9):4310. doi: 10.3390/ijms22094310. Int J Mol Sci. 2021. PMID: 33919221 Free PMC article.
-
Platelet Lysate: The Better Choice for Jaw Periosteal Cell Mineralization.Stem Cells Int. 2017;2017:8303959. doi: 10.1155/2017/8303959. Epub 2017 Dec 17. Stem Cells Int. 2017. PMID: 29391870 Free PMC article.
-
Raman Spectroscopic Analyses of Jaw Periosteal Cell Mineralization.Stem Cells Int. 2017;2017:1651376. doi: 10.1155/2017/1651376. Epub 2017 Jan 23. Stem Cells Int. 2017. PMID: 28232849 Free PMC article.
-
Mechanism of Bone Mineralization.Cold Spring Harb Perspect Med. 2018 Dec 3;8(12):a031229. doi: 10.1101/cshperspect.a031229. Cold Spring Harb Perspect Med. 2018. PMID: 29610149 Free PMC article. Review.
-
Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models.Biochim Biophys Acta Gen Subj. 2018 Mar;1862(3):532-546. doi: 10.1016/j.bbagen.2017.11.005. Epub 2017 Nov 3. Biochim Biophys Acta Gen Subj. 2018. PMID: 29108957 Free PMC article. Review.
Cited by
-
Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis.Int J Mol Sci. 2024 Feb 16;25(4):2355. doi: 10.3390/ijms25042355. Int J Mol Sci. 2024. PMID: 38397031 Free PMC article.
-
Injection of Porcine Adipose Tissue-Derived Stromal Cells by a Novel Waterjet Technology.Int J Mol Sci. 2021 Apr 12;22(8):3958. doi: 10.3390/ijms22083958. Int J Mol Sci. 2021. PMID: 33921246 Free PMC article.
-
Jaw Periosteum-Derived Mesenchymal Stem Cells Regulate THP-1-Derived Macrophage Polarization.Int J Mol Sci. 2021 Apr 21;22(9):4310. doi: 10.3390/ijms22094310. Int J Mol Sci. 2021. PMID: 33919221 Free PMC article.
-
5-Aminolevulinic Acid-Mediated Photodynamic Therapy Potentiates the Effectiveness of Doxorubicin in Ewing Sarcomas.Biomedicines. 2022 Nov 11;10(11):2900. doi: 10.3390/biomedicines10112900. Biomedicines. 2022. PMID: 36428464 Free PMC article.
-
Mechanical and Functional Improvement of β-TCP Scaffolds for Use in Bone Tissue Engineering.J Funct Biomater. 2023 Aug 16;14(8):427. doi: 10.3390/jfb14080427. J Funct Biomater. 2023. PMID: 37623671 Free PMC article.
References
-
- De Bari C., Dell’Accio F., Vanlauwe J., Eyckmans J., Khan I.M., Archer C.W., Jones E.A., McGonagle D., Mitsiadis T.A., Pitzalis C., et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. - DOI - PubMed
-
- Bruder S.P., Jaiswal N., Haynesworth S.E. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

