N-Heterocyclic Carbene-Platinum Complexes Featuring an Anthracenyl Moiety: Anti-Cancer Activity and DNA Interaction
- PMID: 31461928
- PMCID: PMC6747518
- DOI: 10.3390/ijms20174198
N-Heterocyclic Carbene-Platinum Complexes Featuring an Anthracenyl Moiety: Anti-Cancer Activity and DNA Interaction
Abstract
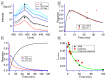
A platinum (II) complex stabilized by a pyridine and an N-heterocyclic carbene ligand featuring an anthracenyl moiety was prepared. The compound was fully characterized and its molecular structure was determined by single-crystal X-ray diffraction. The compound demonstrated high in vitro antiproliferative activities against cancer cell lines with IC50 ranging from 10 to 80 nM. The presence of the anthracenyl moiety on the N-heterocyclic carbene (NHC) Pt complex was used as a luminescent tag to probe the metal interaction with the nucleobases of the DNA through a pyridine-nucleobase ligand exchange. Such interaction of the platinum complex with DNA was corroborated by optical tweezers techniques and liquid phase atomic force microscopy (AFM). The results revealed a two-state interaction between the platinum complex and the DNA strands. This two-state behavior was quantified from the different experiments due to contour length variations. At 24 h incubation, the stretching curves revealed multiple structural breakages, and AFM imaging revealed a highly compact and dense structure of platinum complexes bridging the DNA strands.
Keywords: AFM and optical tweezers microscopy; N-heterocyclic carbene; antitumoral activity; platinum; two-state interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Rougier P., Zarba J.J., Ducreux M., Basile M., Pignon J.P., Mahjoubi M., Benahmed M., Droz J.P., Cvitkovic E., Armand J.P. Phase II study of cisplatin and 120-hour continuous infusion of 5-fluorouracil in patients with advanced pancreatic adenocarcinoma. Ann. Oncol. 1993;4:333–336. doi: 10.1093/oxfordjournals.annonc.a058495. - DOI - PubMed
-
- Williams S.D., Stablein D.M., Einhorn L.H., Muggia F.M., Weiss R.B., Donohue J.P., Paulson D.F., Brunner K.W., Jacobs E.M., Spaulding J.T. Immediate adjuvant chemotherapy versus observation with treatment at relapse in pathological stage II testicular cancer. N. Engl. J. Med. 1987;317:1433–1438. doi: 10.1056/NEJM198712033172303. - DOI - PubMed
-
- Alberts D.S., Liu P.Y., Hannigan E.V., O’Toole R., Williams S.D., Young J.A., Franklin E.W., Clarke-Pearson D.L., Malviya V.K., DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

